7.1: Orbitals of Polyelectronic Atoms
- Page ID
- 52823
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
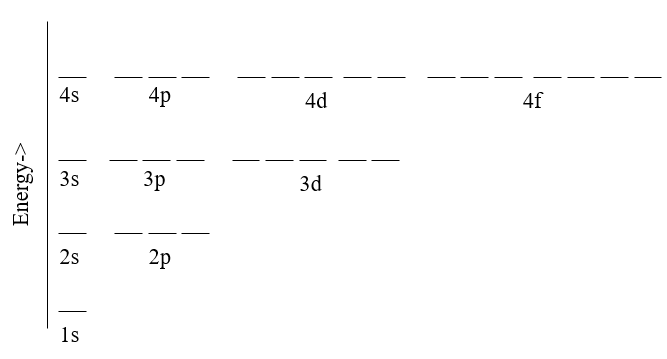
In the previous Chapter we learned that there are three types of orbitals for the Schrödinger wave equation solution of the hydrogen like atom that can be described by the three quantum numbers; n, l and ml. We also learned that an electron in an orbital can have one of two spin states, which provided a fourth quantum number, the spin quantum number ms. When there is only one electron the only interaction is between the electron and the nucleolus and so all the energies of a each principle quantum number are degenerate, that is of the same energy (left side of Figure \(\PageIndex{1}\)). It is because of this that the Bohr model works for hydrogen atom, and each principle quantum number is often referred to as an electron shell, and in this text we will use these terms interchangeably. Once you have a multi-electron system though, you start to get electron - electron interactions in addition to electron-nucleus interactions, and all of these interactions are influenced by the shapes and of the orbitals. And so in the multi-electron system there is a lifting of the degeneracy of the azimuthal orbitals and the Bohr model no longer works (left side of Figure \(\PageIndex{1}\)).
 |
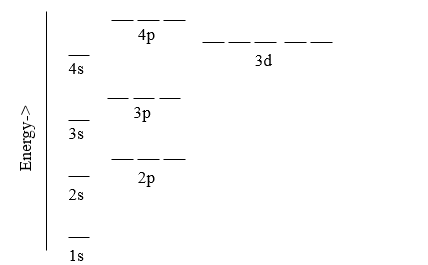 |
| Figure \(\PageIndex{1}\): For hydrogen where there is only one electron all the azimuthal orbitals are degenerate (of equal energy) and so the Bohr model works. For the multielectron atom there are electron-electron interactions in addition to electron-nucleus interactions and these are influenced by the shapes of the orbitals and so the Bohr model breaks down due to a lifting of the degeneracy of the azimuthal orbitals. | |
This results in the different blocks of the periodic table (Figure \(\PageIndex{1}\). In the presence of an external magnetic field there is a further lifting of the degeneracy of the magnetic quantum numbers (ml), with some showing no change in the energy of the azimuthal orbital (ml=0), some showing an increase in energy as they align with the external magnetic field (ml=+1, +2 ....), and others showing a decrease in energy as there align against the external magnetic fields (ml=-1, -2, ...). It should be noted that due to conservation of energy, the amount of energy gained by the positive ml is of the same magnitude as the amount lost by the respective negative ml. This can be observed in the line spectra of the atoms when they are in the presence of an external magnetic field, and the magnitude of the split is dependent on the strength of the external field.
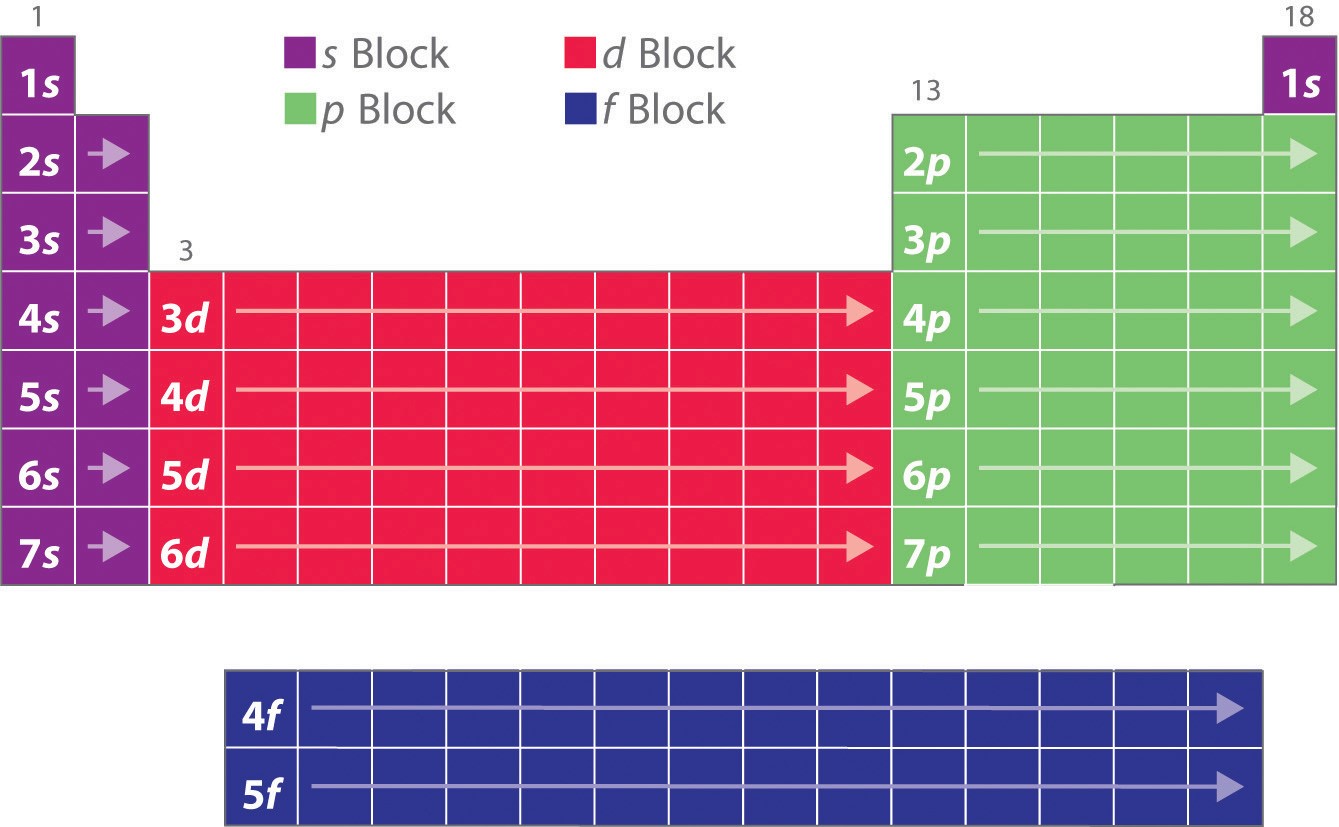
Periodic Table and Orbitals
The structure of the periodic table actually depends on the types of orbitals being filled with electrons for elements in the lowest energy electron configurations, which we call the ground state. We can define different regions by the types of orbitals electrons reside in, for example the alkali and alkaline earth metals are often referred to as the s-block as the outer (valence) shell electrons are filling the s orbitals of that shell (principle quantum number). So for Li and Be one and two electrons are filling the 2s orbital, while for Na and Mg they are filling the 3s orbitals. It should be noted that when you have more than one electron in an orbital they must have opposite spins (different spin quantum numbers). For these region each orbital can hold up to two electrons. Figure \(\PageIndex{1}\) shows the different blocks based on the azimuthal quantum numbers. Note, as discussed in section 6.5.5, the first s orbital is in the n=1 shell, the first p orbital is in the n=2 shell, the first d orbital is in the n=3 and the first f orbital is in the n=4 shell, where we are using the concept of "shell" to align with the principle quantum number. Also note, there are 1 type of s, 3 types of p, 5 types of d and 7 types of f orbitals, and as discussed above, since there are two spin quantum states (numbers) for each orbital, there can be up to two electrons in an orbital. Because of this there are two atoms for each shell in the s block, 6 atoms in the p block (that has 3 orbitals), 10 atoms in the d block (that has 5 orbitals) and 14 atoms in the f block (that has 7 orbtials).
On closer inspection it can be noted that the 4s orbitals are filled before the 3d. Why is this, when electrons in shells of higher the principle quantum numbers are further away from the nucleus, and so feel less of an attraction? While it is true that a 4s orbital is further away from the nucleus, it is also closer. To understand this we must remember that an electron has wave-particle duality and we need to look at its radial probability function \(\psi^2\) (Figure \(\PageIndex{3}\)). What you see is that the 4s orbital has 3 nodes with 4 regions where there is a probability the electron can occur and the inner lobe is closer to the nucleus than the corresponding probability function for the d orbital. But note, most of the 4s orbital is farther away from the nucleus and so its average "radius" is farther away from the nuclues than the 3d.

Figure \(\PageIndex{3}\): radial distribution function shows 4s orbital penetrating 3d, and so an electron in the 4s orbital is both nearer and further from the nucleus than a 3d. (image cc 4.0 from chemistry stack exchange uploaded by orthocresol)
Now things get complicated real quick as for example 6p will penetrate a 4f, and a 7s penetrates both 5f and 6d orbitals.
n+1 Rule: Order of increasing energy for subshells. The order of filling orbitals is they go in order of increasing n+l value (remember l=0 for s, not one), and if two orbitals have the same value like 2p (2+1=3) and 3s (3+0=3), the one with the lower n is filled first. Fortunately there is an easy mnemonic device for remembering both which orbitals exist and their energies. In section 6.5.5 we built a table like that of Figure \(\PageIndex{4}\) and then moving diagonally downwards we come up with the way the orbitals are filled in the periodic table. So you see that the 4s are filled before the 3d, and for example the 5p is filled before the 4f.

Figure \(\PageIndex{4}\): Mnemonic device for memorizing the energies of the orbitals by following the diagnols of the arrows as displayed.
Note how the color coding of the blocks in Figure \(\PageIndex{4}\) correlates to the structure of the periodic table as indicated in Figure \(\PageIndex{2}\)
Exercise \(\PageIndex{1}\)
Rank the following subshells in order of increasing energy, 3d,4s,4f,6s & 5d
- Answer
-
3d = (3+2) = 5
4s = (4+0) = 4
4f = (4+3) = 7
6s = (6+0) = 6
5d = (5+2)=7So: 4s<3d<6s<4f<5d note, both 4f and 5d have n+l values = 7, so 4f is filled first because it is the lower principle quantum number.
Representing Orbital Occupancies
In section 6.5.3 we introduced the convention for naming orbitals where the orbital represented by \(\psi^2_{1s}\) would be represented by 1s, and now we used post superscripts to indicate the number of electrons in an orbital. Figure \(\PageIndex{5}\) shows the ground state configuration of hydrogen, which would have only one electron in a 1s orbital
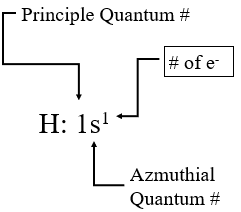
Figure \(\PageIndex{5}\): Convention for showing number of electrons in an orbital.
If the magnetic quantum number or the specific azimuthal orbital is indicated then the maximum number of electrons that can be in an orbital is two, but if just the azimuthal orbital is indicated, you can have more than two electrons (up to 6 for p, up to 10 for d and up to 14 for f orbitals). For example 4p5 means we have 5 electrons in the three 4p orbitals (px, py and pz) of which two orbitals have paired electrons of opposite spin and one has a lone unpaired electron. If we wrote 4p5x it would be wrong and nonsense, as according to the Pauli Exclusion principle (section 6.7.3) there can only be one or two electrons in the px orbital, and they must have opposite spin.
So 4p5 implies one of three possible configurations, where the unpaired electron is expressed in red:
\[ 4p^2_x4p^2_y{\color{Red} 4p_z} \\ \;\\ or \\ \; \\ 4p^2_x{\color{Red} 4p_y} 4p_z^2 \\ \; \\or \\ \; \\ {\color{Red} 4p_x} 4p^2_y4p_z^2 \nonumber\]
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
Some material adopted, modified or adapted from
- anonymous contributors to LibreText


