6.3: Atomic Line Spectra and Niels Bohr
- Page ID
- 52785
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Define the ground state ground state and excited state of electrons
- Explain the difference between line spectra for absorption and emission of electrons
- Calculate the energy associated with the electron at specified energy state
- Calculate the energy difference with electron transition from a higher energy to lower energy, and vice versa
- Calculate the wavelength of light emitted as a result of a particular energy transition
- Differentiate between the Balmer, Lyman, and Paschen Series
- Describe the limitation of the Bohr Model
Line Spectra
There are two types of line spectra, emission and absorption. In an emission spectra electrons are excited to an excited state by thermal or electrical means and then relax back to a lower state and emit a photon of light at a specific energy, which is seen as at a specific wavelength. That is, electrons transfer from high to low energy and give off a photon of light with an energy equal to the difference in energy between the two electronic states. In an absorption spectra the opposite occurs and a sample is radiated with white light of multiple wavelengths, and if there is a wavelength of the same energy as the energy gap between two electronic states, that photon can be absorbed as an electron goes from the lower to higher (excited) energy state. Emission is an exothermic process in that a photon of energy leaves the system, while absorption is an endothermic process as a photon of energy is gained by the system.
Atomic Absorption Line Spectra
The following YouTube of the Sodium Absroption Lines from the Harvard Natural Sciences Lecture Demonstrations shows how an absorption line can be seen with rather primitive equipment.
In video \(\PageIndex{1}\) you see what appears to be a single line but there are actually two lines known as the Sodium D-lines, at 588.9950 and 589.5924 nm. What is clear is that with very low technology these lines can be seen, and where they occur is unique for each element.
Hydrogen's absorption line spectra is shown in Figure \(\PageIndex{1}\). Here we see five black lines indicating that light at these wavelengths is being absorbed. The colored background is light that was transmitted through the sample because there was no allowed transition where the energy gap between two orbitals equaled the energy of the photon (h\(\nu\)) .
| \(\lambda= 656nm \; \; \lambda=486nm \\ \lambda=434nm \; \ ;\lambda= 410nm \) |
Figure \(\PageIndex{1}\): Hydrogen Absorption Spectra, as would be observed if a continuous spectra was passed through hydrogen gas that was not excited, along with the 4 visible wavelengths (the line on the far left is at 397nm not really visible)
Atomic Emission Line Spectra
In a gas discharge tube energy is added to a gas which is adsorbed by electrons. These excited electrons enter high energy orbitals and then fall back to their lower energy and can give off a photon of light of a specific wavelength that can be seen by a diffraction grating or prism. These type of spectra are called line spectra (in contrast to continuous spectra like the colors of the rainbow) and each element has a unique line spectra that can be used to identify it.
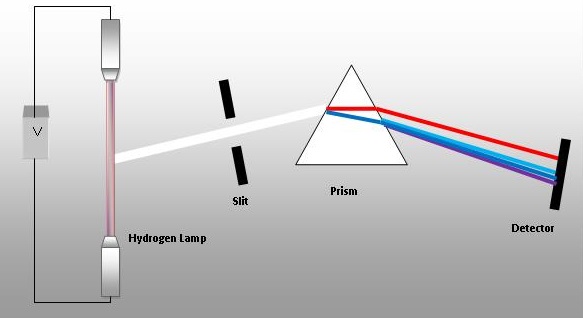
The following YouTube from Flinn Scientific goes over handling a gas discharge tube and shows the line spectra for several
.
| \(\lambda= 656nm \; \; \lambda=486nm \\ \lambda=434nm \; \ ;\lambda= 410nm \) |
Figure \(\PageIndex{3}\): Hydrogen Emission Spectra that would be observed if the light from a gas discharge tube filled with hydrogen was passed through a filter or prisim.
Before proceeding Figures \(\PageIndex{1}\) and \(\PageIndex{3}\) should be compared, and noted that for lines occur at the same wavelengths.
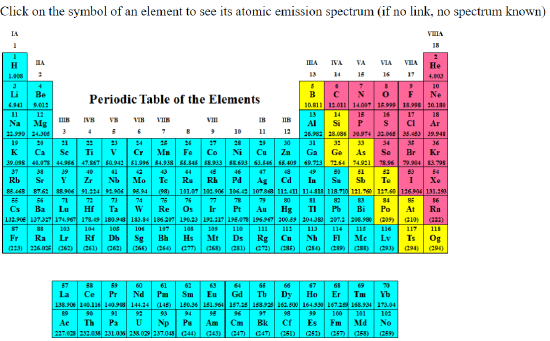
Note: Interactive periodic table with atomic emission spectra
The following image is a link to an interactive periodic table hosted by Alan Jircitano at Penn State Behrend. If you click on an element, on that table it will pull up an emission spectra, if one exits
Spectra of Hydrogen
With only one electron and one proton hydrogen is the simplest atom and early studies of its line spectra lead to some of the first theories with an experimental foundation for our understanding of the atom. The earliest equations were empirical in nature, that is, they dealt with mathematical formulas that could explain experimental observations, even if there was no understanding of why or what was going on. Although unknown in these early times, what we now know is that a photon of light can exchange energy with an electron, with the electron changing orbitals, where each orbital is of a different energy, and it is the difference in energy of the electron orbitals that must equal the energy of an emitted or absorbed photon in a manner that conserves energy.
Balmer Equation
Based on empirical data measured by Anders Angstrom, Johann Balmer (1825- 1898) was a Swiss mathematician who developed an equation for computing the wavelengths of the line spectra. It needs to be emphasized that although this equation could account for the line spectra it was based on empirical (experimental) data.
\[\lambda =B\left ( \frac{n^{2}}{n^{2}-2^{2}} \right )\]
Balmer Equation describing the visible spectrum for hydrogen atom, where B = 364.56 nm, and n is an integer larger than 2.
Rydberg Equation
Johannes Rydberg came up with a more general equation of which the Balmer equation is a specific example for n
\[\frac{1}{\lambda }=R\left(\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right)\]
R is the Rydberg constant, R= 1.097x107m-1 and n1<n2. The following image shows the line spectra in the ultraviolet (Lyman series), visible (Balmer series) and various IR series that are described by the Rydberg equation.
Example \(\PageIndex{1}\): Algebra challenge
Can you derive the Balmer Equation from the Rydberg equation and using the Rydberg constant of R= 1.097x107m-1 come up with a value for the Balmer constant?
Solution
B= 364.5 nm. See video \(\PageIndex{4}\) for the solution of this problem
Bohr Model
The advance of the Bohr model is that it related energy to the observations Rydberg and Balmer's equations described, and it introduced the concept of the quantization of the energy level of an electron in an orbit. Where it failed was that it viewed electrons to reside in a classical orbit that in principle could be defined by a radial distance. Before reading any more watch Video \(\PageIndex{3}\) and note that this video treats the electrons as residing in orbitals, not orbits. Orbitals will be discussed in sections 6.5 and 6.6.
The Bohr model provides a theoretical framework for understanding line spectra. If a photon of light is absorbed, its energy (h\(\nu\)) is transferred to an electron which jumps from a low energy orbit to a high energy orbit, and the absorption spectral lines are correlated to wavelengths associated with the frequency of that light (c=\(\lambda \nu\)). In an emission spectra electrons are excited to upper energy states by some external energy source (thermally or electronically like in a discharge lamp), and then the excited electron spontaneously falls back to the lower energy ground state. This may happen through thermal or radiative means, and if it occurs through a radiative process, a photon of the energy associated with the electronic transition is emitted and this accounts for the lines in an emission spectrum. It is important to recognize that absorption is an endothermic process where the atom gains the energy of the photon, and emission is an exothermic process where the atom losses the energy of the emitted photon.
Figure \(\PageIndex{5}\): Energies of quantized orbitals (left) and absorption and emission transitions of electrons between orbitals (right), with infinity correlating to ionization (removal of an electron from the atom). Those transitions between orbits and that involve the n=1 state are high energy ultraviolet radiation and those involving the n=2 to or from higher n states are in the visible region (the n=2 to n=1 transition is ultravioloet)
The Bohr model is actually very simple to understand, in that it states the energy of the nth orbital is quantized, and inversely related to the square of the quantum number (n) times the energy required to ionize the electron, that is, to remove it from an orbit. This is described by the following equation and the reason it is negative is because zero energy is that when the electron and proton are separated by infinity, and noting that to remove an electron is an endothermic process (you add energy), means the energy of an electron in an orbital must be less than zero..
\[E_{n}=-R_{\infty}\left(\frac{1}{n^{2}}\right)\]
Note: The above equation is a form of an inverse square law, except that unlike other inverse square laws, it is based on the value of an integer, \(\frac{1}{n^{2}}\), giving it values of 1,1/4,/1/9,/16,1/25...
Other inverse square laws are like Coulomb's Law, \(F=k\frac{Q_{1}Q_{2}}{r^{2}}\) and Newton's Law \(F=G\frac{m_{1}m_{2}}{r^{2}}\) . The difference is that the Bohr model is treating energy as a series of discrete (quantized) values inversely proportional to an integer, while Coulomb's and Newton's Law relates the force to the inverse square of the distance between two interacting particles, and is a continuum. That is, unlike Bohr's model, the particles can be separated by any distance (r is not restricted to discrete values).
\[E_{electron}= \Delta E_{n_{i}\rightarrow{n_{f}}}=E_{n_{f}}-E_{n_{i}}=-R_{\infty}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
\[E_{photon}=h\nu =\frac{hc}{\lambda }\]
Absorption Spectrum
(this is endothermic and nf > ni)
The energy of the photon equals the energy of the electron transition as it absorbs the energy and goes to a higher level.
\[E_{photon}=E_{electron}\]
substituting \(E_{photon} =h\nu\)
\[h\nu =E_{n_{f}}-E_{n_{i}}\]
substituting \(\nu = \frac{c}{\lambda} \;from \; c=\lambda\nu\) (eq. 6.1.2)
\[\frac{hc}{\lambda }=-R_{\infty}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
Noting \(R_{\infty}\) is the minimum energy required to photo-ionize an electron in the lowest energy level, that is, to eject the electron from hydrogen so it is not longer in an orbital.
\[\frac{hc}{\lambda }=-R_{\infty}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
To derive the Rydberg Equation we first divide by hc
\[\frac{1}{\lambda }=-\frac{R_{\infty}}{hc}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
Then multiply by negative one
\[\frac{1}{\lambda }=\frac{R_{\infty}}{hc}\left(\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right)\]
And equate the Rydberg constant to \(R_{\infty}\)/hc
\[R=\frac{R_{\infty}}{hc}\]
gives the Rydberg Equation
\[\frac{1}{\lambda }=R\left(\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right)\]
Note R = 1.097x107m-1
and is the historical Rydberg constant that predicted the wavelength of the hydrogen spectra.
What we are calling \(R_{\infty}\) is the minimum energy required to remove an electron from the ground state (lowest energy) orbital of hydrogen, and
\(R_{\infty}\) = 2.18x10-18J
Note
The symbol R is often used to represent both the Rydberg constant and \(R_{\infty}\) and to know which is being defined you need to look at the units.
- If Given R = 1.097x107m-1, use
\[\frac{1}{\lambda }=R\left(\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right)\]
- If Given R = 2.18x10-18J
\[\frac{1}{\lambda }=-\frac{R}{hc}\left(\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right)\]
noting that hc has units of \( \left (J \cdot sec \right )\left ( \frac{m}{sec} \right )=J \cdot m\)
Emission spectrum
In emission the system loses an energy as the photon is leaving (exothermic) and so the energy of the photon is negative (ni > nf)
\[-E_{photon}=E_{electron}\]
\[-\frac{hc}{\lambda }=-R_{\infty}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
Noting \(R_{\infty}\) is the minimum energy required to photo-ionize an electron in the lowest energy level, that is, to eject the electron from hydrogen so it is not longer in an orbital.
\[\frac{hc}{\lambda }=R_{\infty}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
To derive the Rydberg Equation we first divide by hc
\[\frac{1}{\lambda }=\frac{R_{\infty}}{hc}\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
or
\[\frac{1}{\lambda }=R\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\]
Note
If you are solving for a wavelength and you get a negative wavelength, you probably treated emission as if it was absorption, as there is no such thing as a negatative wavelength.
Practice Problems
Example \(\PageIndex{2}\)
Using the Bohr model calculate the excited state an electron relaxes from for the blue line of the hydrogen emission spectra at \(\lambda 486\) as seen in Figure 6.3.3.
Solution
For emission we know that \[-E_{photon}=E_{electron}\] and for visible radiation the final state is the n=2 state.
Using eq. 6.3.20 and solving for ni gives:
\[n_i=\sqrt{\frac{1}{\frac{1}{n_{f}^2}-\frac{1}{\lambda R}}}\]
noting: \(R=1.097m^{-1}=0.01097nm^{-1}\) and ni=2 for visible radiation
gives
\[n_i=\sqrt{\frac{1}{\frac{1}{2^2}-\frac{1}{486nm\left ( 0.01907nm^{-1} \right )}}}=4\]
And so the blue line is for light emitting from an electronic transition from the n=4 to n=2 state.
Exercise \(\PageIndex{1}\)
What is the final quantum level in the absorption spectrum of Figure 3.6.1 for the purple band at 434 nm?
- Answer
-
For absorption we know that \[E_{photon}=E_{electron}\] and noting for visible radiation the initial state is the n=2 state.
Using eq. 6.3.13 and noting for nf.
\[n_f=\sqrt{\frac{1}{\frac{1}{n_{i}^2}-\frac{1}{\lambda R}}}\]
noting: \(R=1.097m^{-1}=0.01097nm^{-1}\) and ni=2 for visible radiation
gives
\[n_f=\sqrt{\frac{1}{\frac{1}{2^2}-\frac{1}{434nm\left ( 0.01907nm^{-1} \right )}}}=5\]
And so the purple absorption band at 434 nm is for light being absorbed as an electron jumps from the n=2 to the n=5 state.
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Ronia Kattoum (UALR)
- Material adopted and adapted from Paul Flowers, et al. Open Stax
- anonymous