2.2: Isotopes: Atomic Number and Atomic Mass Unit
- Page ID
- 50427
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Differentiate between atomic number and mass number
- Define isotopes
- Compare characteristics of varying isotopes
- Define the atomic mass unit
- Convert between amu’s and grams
Atomic Number and Mass Number
When you study the periodic table, the first thing that you may notice is the number that lies above the symbol. This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element.
The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons. If you change the atomic number to 12, you are no longer dealing with sodium atoms, but magnesium atoms. Hence, the atomic number defines the element in question.
Recall that the nuclei of most atoms contain neutrons as well as protons. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemical behavior. Because different isotopes of the same element haves different number of neutrons, each of these isotopes will have a different mass number(A), which is the sum of the number of protons and the number of neutrons in the nucleus of an atom.
Mass Number(A) = Number of Protons + Number of Neutrons
The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. An isotope of any element can be uniquely represented as AZX, where X is the atomic symbol of the element, A is the mass number and Z is the atomic number. The isotope of carbon that has 6 neutrons is therefore 126C. The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies Z. Consequently, it is more often written as 12C, which is read as “carbon-12.” Nevertheless, the value of Z is commonly included in the notation for nuclear reactions because these reactions involve changes in Z.
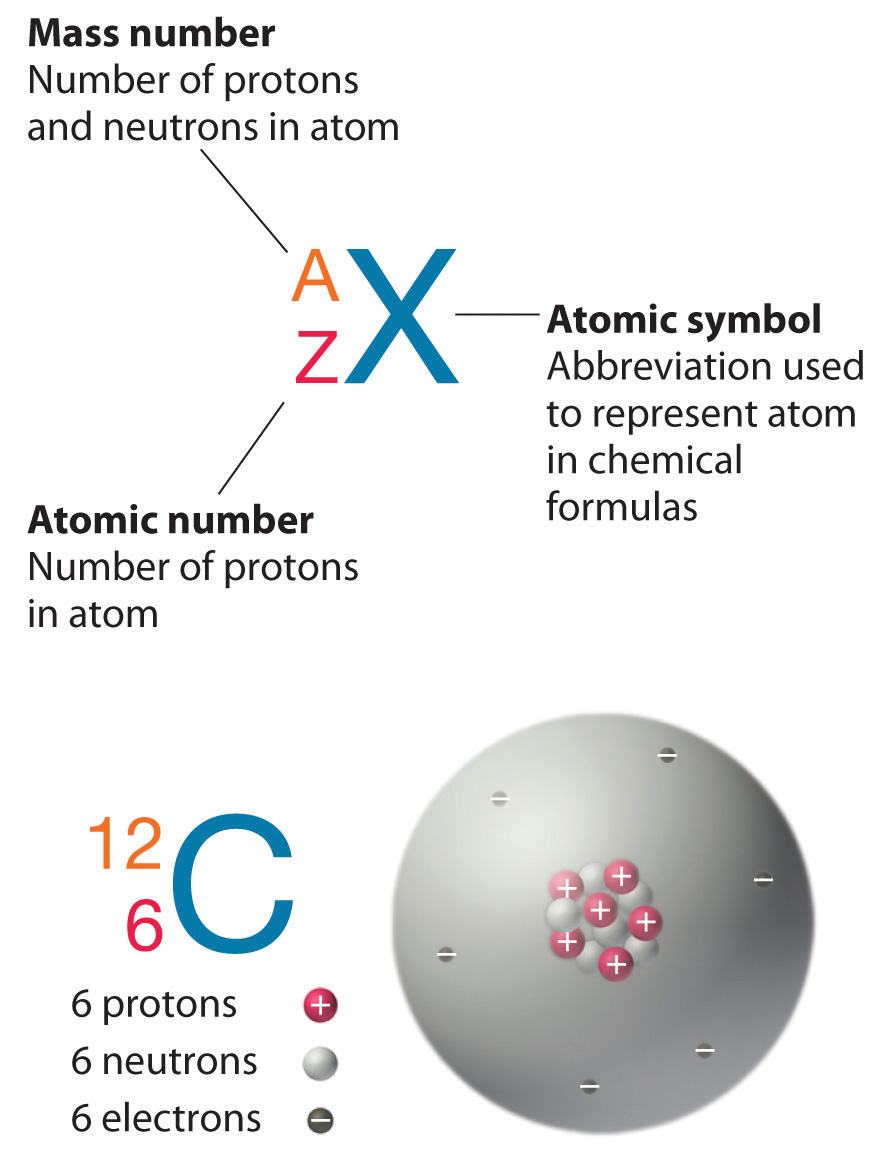
Atomic Mass Unit (Dalton)
The atomic mass unit (u, amu or Da) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amu's (u's). This is the common unit for atomic or molecular mass, and 1 amu is thus 1/12th the mass of a 12C atom. Using this definition, and relating it to the standard mass of the kilogram, one sees the obvious, that the amu is a very small number.
1 amu = 1.66054x10-27Kg = 1.66054x10-24g = 1.66054 yoctograms
The amu is also called the Dalton and large molecules like proteins, nucleic acid and large polymers are often expressed in Kilo Daltons (1kDA=103 Da) or Mega Daltons (1MDa=106 Da).
As a result of this definition, the mass of all other elements on the periodic table are determined relative to carbon-12.
Isotopes
Isotopes are different atoms of the same element that contain different numbers of neutrons in their nucleus, and therefore they have different atomic masses. There are essentially two types of isotopes, stable isotopes and radioactive isotopes. A radioactive isotope or radioisotope is an isotope that undergoes a radioactive decay where the number of protons changes over time and when this occurs an atom of one element is transmuted into another element. This is called a nuclear transmutation. Three types of radioactivity were described in the previous section, \(\alpha\), \(\beta\) amd \(\gamma\) (section 2.1.3). When an atom undergoes \(\alpha\) decay it looses two protons and when it undergoes \(\beta\) decay it gains a proton, and thus these two types of radiation cause nucelear transmutations. \(\gamma\) radiation does not affect the elemental identity of an atom. The rate at which a radioisotope decays is often described by its half life, which is the amount of time it takes for half of a sample to undergo a nuclear transmutation. There are more than 3,000 natural and artificial known isotopes, with many of them having short half lives (less than a month). Note, some radioisotopes have very long half lives (billions of years) and so are essentially stable on human time lines.
Example 2.2.1
An element with three stable isotopes has 82 protons. The separate isotopes contain 124, 125, and 126 neutrons. Identify the element and write symbols for the isotopes.
Given: number of protons and neutrons
Asked for: element and atomic symbol
Strategy:
- Refer to the periodic table and use the number of protons to identify the element.
- Calculate the mass number of each isotope by adding together the numbers of protons and neutrons.
- Give the symbol of each isotope with the mass number as the superscript and the number of protons as the subscript, both written to the left of the symbol of the element.
Solution:
A The element with 82 protons (atomic number of 82) is lead: Pb.
B For the first isotope, A = 82 protons + 124 neutrons = 206. Similarly, A = 82 + 125 = 207 and A = 82 + 126 = 208 for the second and third isotopes, respectively. The symbols for these isotopes are 20682Pb, 20782Pb, and 20882Pb, which also can also be symbolized as Pb-206, Pb-207, and Pb-208.
Exercise \(\PageIndex{1}\)
What is the mass number of a phosphorous atom with 16 neutrons
- Answer
-
31
Exercise \(\PageIndex{2}\)
How many protons, neutrons, and electrons are in As-74 atom?
- Answer
-
33 protons, 41 neutrons, 33 electrons
Exercise \(\PageIndex{3}\)
The actual mass of As-74 is 73.924 amu's.
A) What is the mass of As-74 in grams?
B) What is the mass of the arsenic atom relative to the carbon-12 atom?
- Answer
-
A) Mass of As-74 = (73.924 amu) x (1.66054 x 10-24 g/amu) = 1.2275 x 10-22 g
B) Mass of As-74/Mass of C-12 = 73.924 amu/12.000 amu= 6.1603 ... so, the mass of an As-74 atom is 6,1603 times more than a C-12 atom.
Vocabulary
Test Yourself
Query \(\PageIndex{1}\)
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Anonymous
- Modified by Joshua Halpern, Scott Sinex and Scott Johnson
- November Palmer & Ronia Kattoum(UALR)
- Elena Lisitsyna (H5P interactive modules)


