8.5: Polysaccharides
- Page ID
- 165310
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Polysaccharides, as their name implies, are made by joining together many sugars. The functions for polysaccharides are varied. They include energy storage, structural strength, and lubrication. Polysaccharides involved in energy storage include the plant polysaccharides, amylose and amylopectin. The polysaccharide involved in energy storage in animals is called Glycogen and it is mostly found in the muscles and liver.
Amylose/Amylopectin
Amylose is the simplest of the polysaccharides, being comprised solely of glucose units joined in an alpha 1-4 linkage. Amylose is broken down by the enzyme alpha-amylase, found in saliva. Amylopectin is related to amylose in being composed only of glucose, but it differs in how the glucose units are joined together. Alpha 1-4 linkages predominate, but every 30-50 residues, a ‘branch’ arises from an alpha 1-6 linkage. Branches make the structure of amylopectin more complex than that of amylose.

Glycogen
Glycogen is a polysaccharide that is physically related to amylopectin in being built only of glucose and in having a mix of alpha 1-4 and alpha 1-6 bonds. Glycogen, however, has many more alpha 1-6 branches than amylopectin, with such bonds occurring about every 10 residues. One might wonder why such branching occurs more abundantly in animals than in plants. A plausible explanation is based on the method by which these molecules are broken down. The breakdown of these polysaccharides is catalyzed by enzymes, known as phosphorylases, that clip glucose residues from the ends of glycogen chains and attach a phosphate to them in the process, producing glucose-1-phosphate. More highly branched polysaccharides have more ends to clip, and this translates to more glucose-1-phosphates that can be removed simultaneously by numerous phosphorylases. Since glucose is used for energy by muscles, glucose concentrations can be increased faster the more branched the glycogen is. Plants, which are immobile do not have needs for such immediate release of glucose and thus have less need for highly branched polysaccharides.
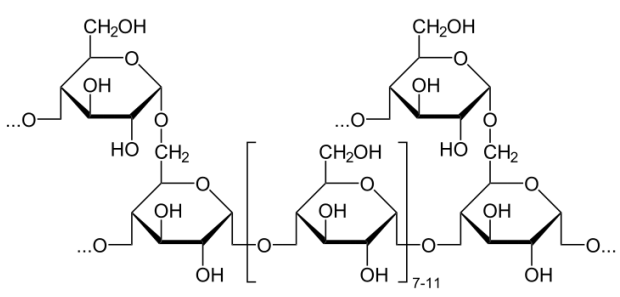
Cellulose
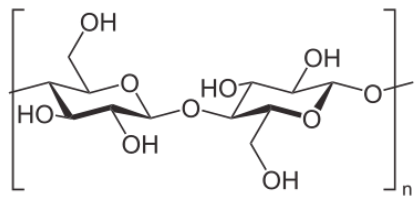
Another important polysaccharide containing only glucose is cellulose. It is a polymer of glucose used to give plant cell walls structural integrity and has the individual units joined solely in a beta 1-4 configuration. That simple structural change makes a radical diff erence in its digestibility. Humans are unable to break down cellulose and it passes through the digestive system as roughage. Ruminant animals, such as cattle, however have bacteria in their rumens that contain the enzyme cellulase. It breaks the beta 1-4 links of the glucoses in cellulose to release the sugars for energy.
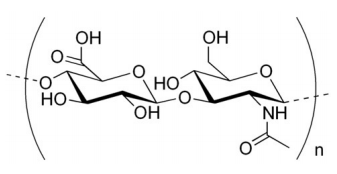
Another polysaccharide used for structural integrity is known as chitin. Chitin makes up the exoskeleton of insects and is a polymer of a modified form of glucose known as N-acetyl-glucosamine.
Glycosaminoglycans
Yet another category of polysaccharides are the glycosaminoglycans (also called mucopolysaccharides ), some examples of which include keratan sulfate, heparin, hyaluronic acid, and chondroitin sulfate. The polysaccharide compounds are linked to proteins, but differ from glycoproteins in having a much larger contingent of sugar residues and, further, the sugars are considerably more chemically modified. Each of them contains a repeating unit of a disaccharide that contains at least one negatively charged residue. The result is a polyanionic substance that, in its interactions with water, makes for a “slimy" feel. Glycosaminoglycans are found in snot, and in synovial fluid, which lubricates joints. Heparin is a glycosaminoglycan that helps to prevent blood from clotting.
Contributors
Dr. Kevin Ahern and Dr. Indira Rajagopal (Oregon State University)
Thumbnail: Schematic two-dimensional cross-sectional view of glycogen: A core protein of glycogenin is surrounded by branches of glucose units. The entire globular granule may contain around 30,000 glucose units. By Mikael Häggström, used with permission (Public Domain).

