5.13: Nucleophilic Addition of Amines- Imine and Enamine Formation
- Page ID
- 470312
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Reaction with Primary Amines to form Imines
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is eliminated in the reaction.
Predicting the Products of an Imine Forming Reaction
During imine formation, the carbonyl oxygen is completely removed. The nitrogen of the 1o amine reactant replaces the carbonyl oxygen to form the imine C=N bond. During the process the nitrogen of the 1o amine loses both of its hydrogens.
Examples of Imine Forming Reactions
Mechanism of Imine Formation
Imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. Acid protonation of the carbinolamine oxygen converts it into a better leaving group which is subsequently eliminated as water producing an iminium ion. Deprotonation of nitrogen gives the final imine product.
1) Nucleophilic addition
2) Protron transfer
3) Protonation
4) Water is eliminated to form an iminium ion.
5) Deprotonation
Reversibility of Imine Forming Reactions
Imines can be hydrolyzed back to the corresponding 1o amine under acidic conditions.
Reactions Involving other Reagents of the type Y-NH2
A wide variety of substances with −NH2 groups can react with aldehydes and ketones by an addition-elimination sequence to yield compounds with a carbon-nitrogen double bond. Imines are sometimes difficult to isolate and purify due to their sensitivity to hydrolysis. Consequently, other reagents of the type Y–NH2 have been studied, and found to give stable products (R2C=N–Y) useful in characterizing the aldehydes and ketones from which they are prepared. Some of these reagents are listed below, together with the structures and names of their carbonyl reaction products. Hydrazones are used as part of the Wolff-Kishner reduction and will be discussed in more detail in Section 19.9.
With the exception of unsubstituted hydrazones, these derivatives are easily prepared and are often crystalline solids - even when the parent aldehyde or ketone is a liquid. Since melting points can be determined more quickly and precisely than boiling points, derivatives such as these are useful for comparison and identification of carbonyl compounds. It should be noted that although semicarbazide has three nitrogen groups (–NH2) only one of them is a reactive amine. The other two are similar to amides and are deactivated by resonance with the adjacent carbonyl group.
Example
Cinnamaldehyde reacting with hydroxylamine to form cinnamaldehyde oxime
Acetone reacting with semicarbazide to form acetone semicarbazide
The pH Dependence of Imine Forming Reactions
The pH for reactions which form imine compounds must be carefully controlled. The rate at which these imine compounds are formed is generally greatest near a pH of 5, and drops at higher and lower pH's. At high pH there will not be enough acid to protonate the OH in the intermediate to allow for removal as H2O. At low pH most of the amine reactant will be tied up as its ammonium conjugate acid and will become non-nucleophilic.
Schematic variation of the rate of condensation of RNH2 with a carbonyl compound as a function of pH.
Clearly, if the unshared electron pair on the nitrogen of RNH2 is combined with a proton it cannot attack the carbonyl carbon to give the aminoalkanol produced during the mechanism. So at high acid concentration (low pH) we expect the rate and the equilibrium for the overall reaction to be unfavorable.
Dehydration of the aminoalkanol is acid catalyzed; this reaction normally is fast at pH values smaller than 3-4. As the pH is increased above 4, the dehydration step in the mechanism decreases in rate because it requires an acid catalyst. At pH 6 dehydration is the slow step of the mechanism, and at higher pH values it finally becomes too slow to give a useful overall rate of reaction.
Reaction with Secondary Amines to form Enamines
Most aldehydes and ketones react with 2º-amines to give products known as enamines (alkene + amine). It should be noted that, like acetal formation, these are acid-catalyzed reversible reactions in which water is lost. Secondary amines form a distinctly different functional group after nucleophilic addition because they lack the second hydrogen on nitrogen required for imine formation. During this reaction a hydrogen is removed from an adjacent carbon forming a C=C bond.
Predicting the Products of an Enamine Forming Reaction
During enamine formation the carbonyl oxygen is completely removed. The nitrogen of the amine reactant replaces the oxygen to form a N-C bond. During the process the amine loses its lone hydrogen. A hydrogen is removed from a carbon adjacent to the original carbonyl carbon forming a C=C between them.
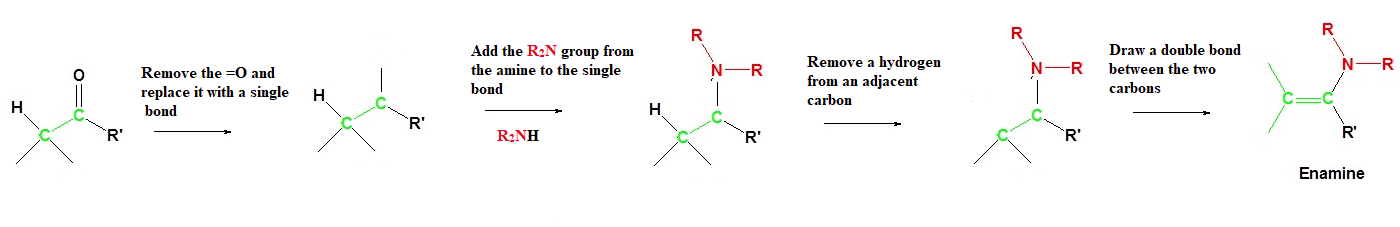
Example
Mechanism
1) The secondary amine undergoes nucleophilic addition to form a neutral tetrahedral intermediate.
2) A proton is transferred from the ammonium ion moiety of the tetrahedral intermediate to the alkoxide ion moiety. This forms a neutral functional group called a carbinolamine.
3) The OH group on the carbinolamine is protonated by hydronium turning it into a good leaving group.
4) The lone pair electrons on the nitrogen form the C=N double bond causing water to be eliminated. This forms an iminium ion
.
5) Water (or a base) removes a hydrogen from an adjacent carbon to form an alkene bond. This pushes two electrons from the C=N double bond onto the positively charged nitrogen creating the neutral enamine product and hydronium (or BH).

