In a previous post, we were introduced to the N-heterocyclic carbenes, a special class of carbene best envisioned as an L-type ligand. In this post, we’ll investigate other classes of carbenes, which are all characterized by a metal-carbon double bond. Fischer carbenes, Shrock carbenes, and vinylidenes are usually actor ligands, but they may be either nucleophilic or electrophilic, depending on the nature of the R groups and metal. In addition, these ligands present some interesting synthetic problems: because free carbenes are quite unstable, ligand substitution does not cut the mustard for metal carbene synthesis.
General Properties
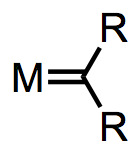
Metal carbenes all possess a metal-carbon double bond. That’s kind of a given. What’s interesting for us about this double bond is that there are multiple ways to deconstruct it to determine the metal’s oxidation state and number of d electrons. We could give one pair of electrons to the metal center and one to the ligand, as we did for the NHCs. This procedure nicely illustrates why compounds containing M=C bonds are called “metal carbenoids”—the deconstructed ligand is an L-type carbenoid. Alternatively, we could give both pairs of electrons to the ligand and think of it as an X2-type ligand. The appropriate procedure depends on the ligand’s substituents and the electronic nature of the metal. The figure below summarizes the two deconstruction procedures.
The proper method of deconstruction depends on the electronic nature of the ligand and metal.
When the metal possesses π-acidic ligands and the R groups are π-basic, the complex is best described as an L-type Fischer carbene and the oxidation state of the metal is unaffected by the carbene ligand. When the ligands are “neutral” (R = H, alkyl) and the metal is a good backbonder—that is, in the absence of π-acidic ligands and electronegative late metals—the complex is best described as an X2-type Schrock carbene. Notice that the oxidation state of the metal depends on our deconstruction method! Thus, we see that even the oxidation state formalism isn’t perfect.
Deconstruction reveals the typical behavior of the methylene carbon in each class of complex. The methylene carbon of Schrock carbenes, on which electron density is piled through backbonding, is nucleophilic (the 2– charge screams nucleophilic!). On the other hand, the methylene carbon of Fischer carbons is electrophilic, because backbonding is weak and does not compensate for σ-donation from the ligand to the metal. To spot a Fischer carbene, be on the lookout for reasonable zwitterionic resonance structures like the one at right below.
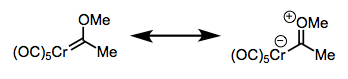
Thanks to the pi-accepting CO ligands, the metal handles the negative charge well. This is a Fischer carbene.
The clever reader may notice that we haven’t mentioned π-acidic R groups, such as carbonyls. Complexes of this type are best described as Fischer carbenes as well, as the ligand is still electrophilic. However, complexes of this type are difficult to handle and crazy reactive (see below) without a π-basic substituent to hold them in check.
Vinylidenes are the organometallic analogues of allenes, and are best described as intermediate in behavior between Fischer and Schrock carbenes. They are electrophilic at the α carbon and nucleophilic at the β carbon—in fact, a nice analogy can be made between vinylidenes and carbon monoxide. Tautomerization to form alkyne π-complexes is common, as the vinylidene and alkyne complexes are often comparable in stability.
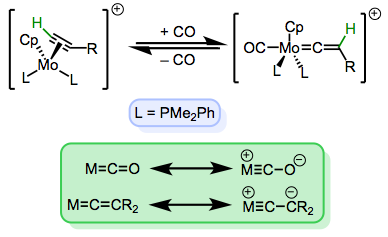
Vinylidene tautomerization, and an analogy between vinylidenes and CO.
Take care when diagnosing the behavior of metal carbenes. In these complexes, there is often a subtle interplay between the R groups on the carbene and other ligands on the metal. In practice, many carbenes are intermediate between the Fischer and Schrock ideals.
Synthesis
Metal carbenes present a fascinating synthetic problem. A cursory look at the deconstruction procedures above reveals that these complexes cannot be made using ligand substitution reactions, because the free ligands are far too unstable. Although the synthetic methods introduced here will be new for us, the attuned organic chemist will find them familiar. The conceptual foundations of metal carbene synthesis are similar to methods for the synthesis of alkenes in organic chemistry.
In the post on NHCs, we saw that the free carbene is both nucleophilic (via the lone pair in its σ system) and electrophilic (via its empty 2pz orbital). Organic precursors to metal carbenes and alkenes also possess this property—they can act both as nucleophiles and electrophiles. Fundamentally, this “ambi-electronic” behavior is useful for the creation of double bonds. One bond comes from “forward flow,” and the other from “reverse flow.” Naturally, the other reacting partner also needs to be ambi-electronic for this method to work.

A fundamental paradigm for double bond synthesis: ambi-electronic compounds doing what they do.
What sets carbene precursors apart from free carbenes? What other kinds of molecules may act as both nucleophiles and electrophiles at the same atom? Watch what happens when we tack a third group onto the free carbene…the figure below shows the result in general and a few specific examples in gray.
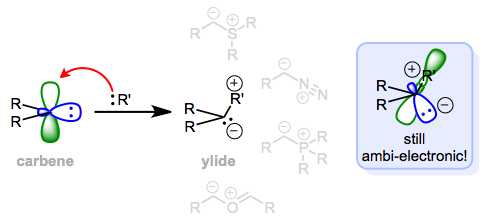
A "dative ligand" R' is the difference between a carbene and an ylide. Both are ambi-electronic.
An ylide, which contains adjacent positive and negative charges, results from this purely hypothetical process. Ylides (diazo compounds, specifically) are the most common precursors to metal carbene complexes. Like free carbenes, ylides are ambi-electronic. The electrophilic frontier orbital of an ylide is just the σ* orbital of the bond connecting the charged atoms, which makes sense if we consider the positively charged fragment as a good leaving group (it always is). The lone pair is still nucleophilic. The figure above depicts some of the most famous ylides of organic chemistry, including those used for alkene synthesis (Corey-Chaykovsky and Wittig) and cycloaddition reactions (the carbonyl ylide).
Although diazo compounds are most commonly drawn with charges on the two nitrogen atoms, the diazo carbon is a good nucleophile and can attack electrophilic metal centers to initiate metal carbene formation. A slick 15N kinetic isotope effect study showed that C–N bond cleavage is the rate-limiting step of the reaction below. Visualize the carbanionic resonance structure to kick off the mechanism! Don’t think too hard about the structure of rhodium(II) acetate here. Rhodium, copper, ruthenium, and iridium all form carbene complexes with diazo compounds in a similar way.

After association of the nucleophilic carbon to Rh, elimination with loss of nitrogen gas is the slow step of this reaction.
Diazo compounds work well for metal carbene formation when they possess an electron-withdrawing group, which stabilizes the ylide through conjugation. What about Fischer carbenes, which possess electron-donating groups on the carbene carbon? An interesting method that still involves a “push-and-pull” of electron flow (but not ylides) employs metal-CO complexes. Upon addition of a strong nucleophile (“forward flow”) to the carbonyl carbon, a metalloenolate of sorts is produced. Treatment with an electrophile RX that prefers oxygen over the metal (“reverse flow”) results in an OR-substituted Fischer carbene. Reactions reminiscent of transesterification trade out the OR group for an –SR group (using a thiol) or an –NR2 group (using a secondary amine).
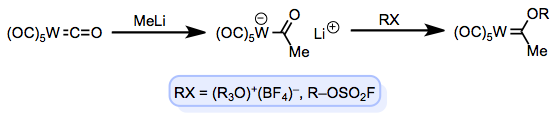
Fischer's classical route to L-type carbenes.
As counterintuitive as it may seem, it’s possible to use metal dianions for the synthesis of Fischer carbenes via a method pioneered by Hegedus and Semmelhack. Potassium intercalated in graphite—the mysterious “KC8“—reduces group 6 metal carbonyl complexes to the corresponding dianions, which subsequently unleash a deluge of electrons on a poor, unsuspecting amide to afford NR2-substituted Fischer carbenes after treatment with trimethylsilyl chloride.
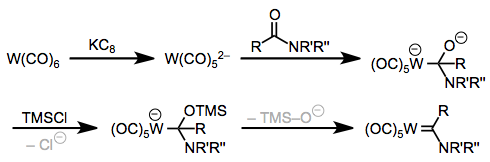
One-directional electron flow for Fischer carbene synthesis: the Hegedus-Semmelhack approach.
For other methods for the synthesis of Fischer carbenes, check out this nice review from the Baran group.
Reactions
In many ways, the reactivity of metal carbenes parallels that of ylides. Olefin metathesis catapulted metal carbenes to international stardom, but in many ways, metathesis is conceptually similar to the Wittig reaction, which employs phosphorus ylides. During both mechanisms, an ylide/carbene hooks up with another doubly bound molecule to form a four-membered ring. This step is followed by what we might call “orthogonal breakdown” to yield two new double bonds.
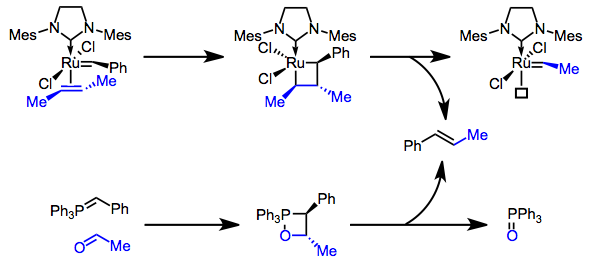
Wittig, Grubbs, and Schrock: Lords of the Chemical Dance. Ambi-electronic molecules are the dancers!
In my opinion, bond insertion reactions are the most interesting processes in which carbenes regularly engage. Bond insertions may be subdivided into cyclopropanation (π-bond insertion) and σ-bond insertion. Evidence suggests that most cyclopropanations take place by a mechanism that overlaps with metathesis—instead of orthogonal breakdown, reductive elimination occurs to release the three carbon atoms as a cyclopropane.
The metallacyclobutane mechanism of cyclopropanation.
σ-Bond insertion involves electron-rich C–H bonds most prominently, suggesting that electrophilic Fischer carbenes should be best for this chemistry. Fischer carbenes incorporating electron-withdrawing groups love to dimerize to form olefins and/or cyclopropanate olefins—how might we put the brakes on this behavior? If we simply tack a π-basic substituent onto the carbene carbon, the reactivity of these complexes is “just right” for C–H insertion. Notably, no covalent organometallic intermediates are involved; the electrophilic carbene carbon snuggles in between C and H in a single step. The transition state of this step resembles the transfer of a hydride from the organic substrate to the carbene, with “rebound” of electron density toward the partial positive charge.
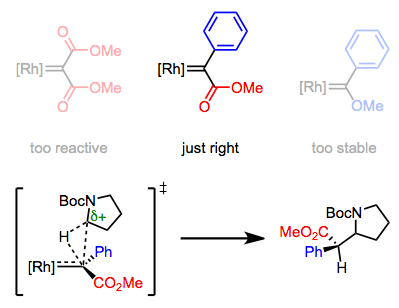
Donor-acceptor carbenoids are the "Goldilocks complexes" of C–H insertion.
Let’s end with a nod to the similarity between Fischer carbenes and carboxylic acid derivatives (esters and amides). Transesterification-type reactions allow the chemist to swap out heteroatomic substituents on the carbene carbon at will (see above). Alkyl substitutents can even be deprotonated at the α carbon, just like esters! When we see the electronic similarities between the M=C bond of a Fischer carbene and the O=C bond of carboxylic acid derivatives, the similar behavior is only natural.


