Dermcidin
- Page ID
- 97957
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
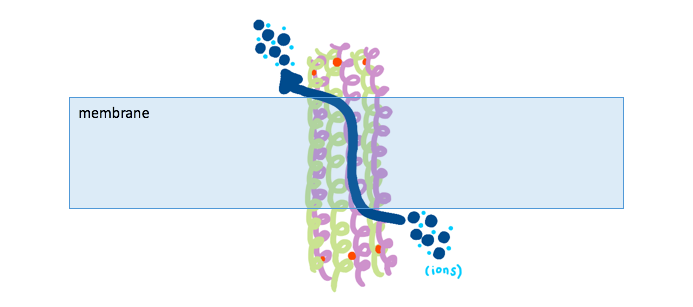
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Dermcidin is an anionic antimicrobial peptide found predominantly in the pores of the human skin, and it is transported through human sweat onto the epidermal surface of the skin.1 Sweat is an aqueous solution that is secreted onto the surface of the skin to aid in cooling off the body, but it also serves other functions including protecting against infection. One of the components that is secreted in sweat is the protein, dermcidin. Demicidin is an antimicrobial peptide that helps to fight off pathogenic bacteria or fungus that are present on the skin. Upon detection of bacteria on the surface of the skin, dermcidin assembles itself, through the aid of Zn2+ ions, into a pore formation (Figure 1). Following insertion into the bacterial membrane, the assembled structure transports ions through its pore and across the bacterial membrane. The transported ions can then inhibit DNA, RNA, and protein synthesis, and additionally inhibit the necessary functionality of ribosomes, chaperone proteins, mitochondria, enzymes, along with cellular respiration, induction of ROS formation, and efflux of ATP and NADH.8 These destructive effects from the transported ions effectively kill the potentially harmful pathogen.9 Humans who lack dermcidin have increased bacterial and fungal infection on the skin, such as atopic dermatitis or tinea pedis. These negative effects due to an overabundance of fungus and bacteria on the skin demonstrate the importance of the antimicrobial function of dermcidin.1
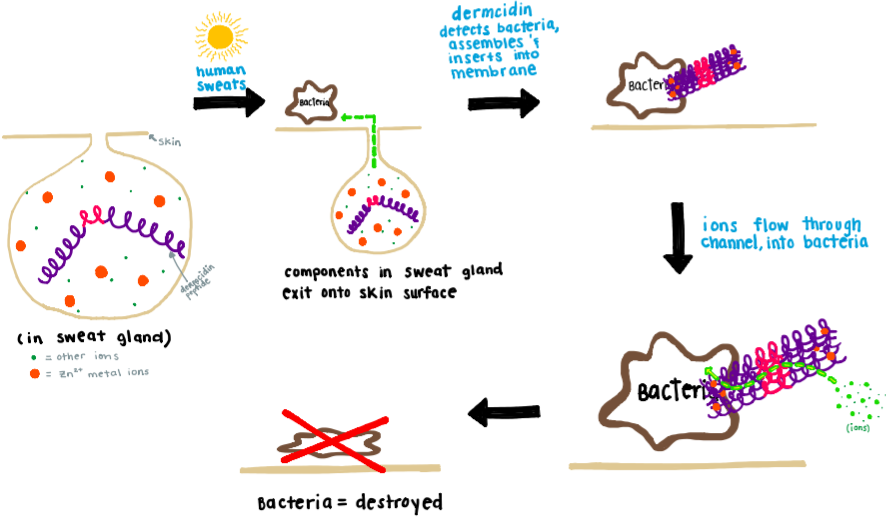
Antimicrobial peptides (AMPs) take advantage of the difference in overall charge between good human cell membranes which contain a neutral charge and negatively charged bacterial cell walls. Bacterial cell walls typically contain many factors which attribute to a net negative charge, such as techoic acids, lipopolysacchirides, and phospholipids.10 Human cell membranes, on the other hand, have a net neutral charge which is actively maintained by ion pumps and channels on the membrane.11 Due to the negative nature of bacterial membranes, most antimicrobial peptides (AMPs) are positively charged, which allows the AMPs to target bacteria specifically.9 Interestingly, dermcidin is not a cationic AMP, but an anionic AMP containing an overall negative charge.9 It is also thought that the rare anionic AMPs evolved in response to bacterial resistance toward cationic antimicrobial peptides (CAMPs). Some bacteria have evolved the strategy of incorporating positively charged polymers into the cell wall, which reduce the cell wall’s otherwise negative charge, making cationic AMPs less likely to target the bacterial cell due to static repulsive forces.4,8 Due to its anionic nature, dermcidin can thus function to target these evolved bacteria. Additionally, it has been found that CAMPs are ineffective in salty fluids, such as sweat in the eccrine gland, therefore anionic AMPs are an alternative and more effective option.4 Although some bacteria have evolved to reduce the negative charge of the cell wall, the overall charge is still slightly negative. Thus, the coordinating Zn2+ metal ions, which are overly abundant in sweat, help to make dermcidin’s charge less negative, allowing dermcidin to effectively target the bacterial membrane.
Dermcidin can be found primarily in two structural conformations depending on its environment. The first structural conformation occurs when Dermcidin is in the skin pore and not in contact with any bacterial membrane or coordinated by Zn2+ ions; it is in an unassembled monomeric helix formation (Figure 2). This monomer of Dermcidin is present in a helix-hinge-helix motif, or “super secondary structure”.2,7
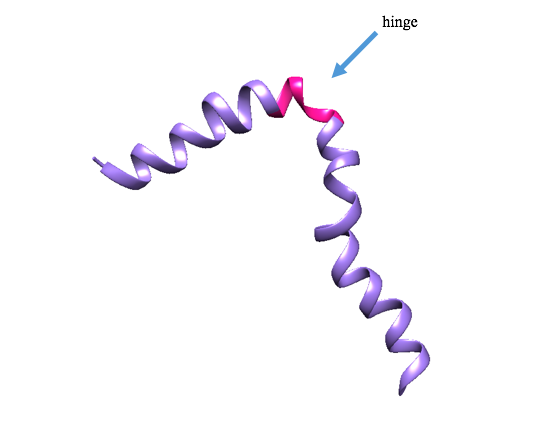
Figure 2: Ribbon depiction of unassembled dermcidin. The unassembled form of dermcidin is shown in its helix-hinge-helix motif. The purple portion is the helix, and the pink portion is the hinge. (PDB: 2ndk)
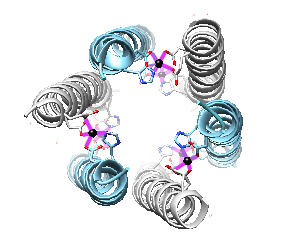
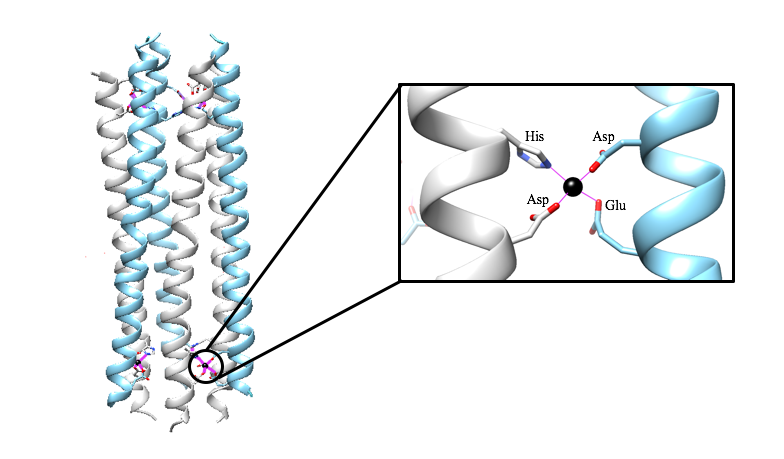
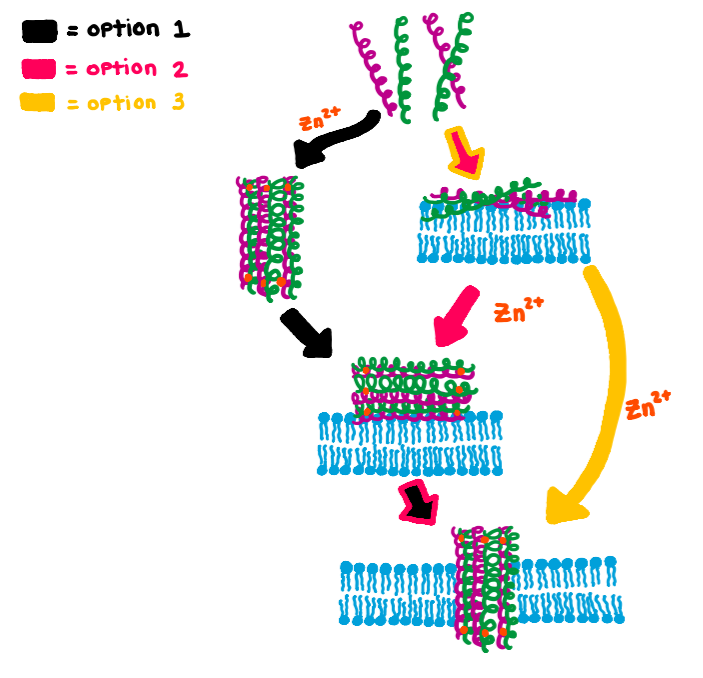
When dermcidin is secreted onto the surface of the skin and comes into contact with bacterial membranes, it assembles into its second structural conformation. A hexameric bundle composed of long alpha-helices is formed by the trimerization of anti-parallel peptide dimers (Figure 3A).2 This hexameric bundle oligomer is stabilized by Zn2+ ions, and its formation results in an enclosed channel structure.2,3 There are two interfaces present; salt-bridges stabilize the extended interface, and the second is stabilized by Zn2+ ions between the two helices of the dimerized peptides.3 As exemplified in Figure 3B, the zinc ions are attached to the inner wall of the channel, and they are coordinated by the charged residues Glutamate (Glu) and Aspartate (Asp), and the neutral residue Histidine (His). The residues are on the N- and C- termini.3 The coordinating residues are selective for Zn2+, as is shown in an experiment where the His residue was mutated to Alanine (Ala). This mutation prevented the dermcidin peptide from assembling into a channel formation, proving that Zn2+ is highly selective toward the His residue.3 The presence of the divalent zinc stabilizes the oligomeric structure of dermcidin when the correct ligands are present (His, Asp, Asp, Glu) and in turn enhance its antimicrobial activity.4 The exact model for dermcidin insertion into the bacterial membrane is not concretely known, although three possibilities have been suggested as can be seen in Figure 4.2
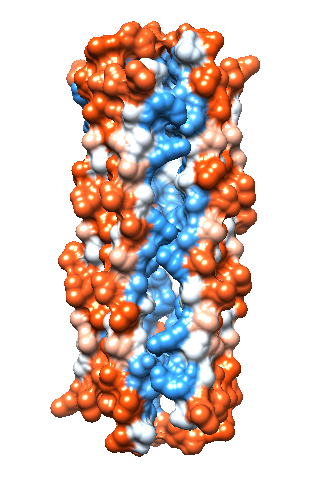
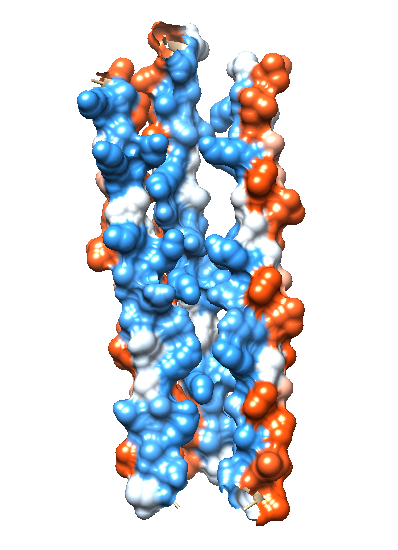
In order for ions to travel across the bacterial membrane, they require a mode of transportation through the hydrophobic bacterial membrane. The assembled dermcidin pore allows ions to penetrate the otherwise impermeable bacterial membrane. Each alpha helix is amphipathic, with one side containing hydrophobic carbon-rich residues, and the other side containing charged hydrophilic residues.9 This allows the outside surface of the dermcidin channel to be hydrophobic (Figure 5A), which allows the the insertion of dermcidin into the likewise hydrophobic bacterial membrane to occur. Figure 5B shows the hydrophilic interior of the dermcidin channel (blue). The hydrophilic interior is necessary in order for charged ions to have a path to travel across the bacterial membrane.
|
A. B. |
|
Figure 5. (A) shows the hydrophobicity of assembled dermcidin and (B) shows a cross section of assembled dermcidin with the red portions on the outer surface exemplifying the hydrophobic areas, and the blue portions exemplifying the hydrophilic areas. (PDB: 2ymk) |
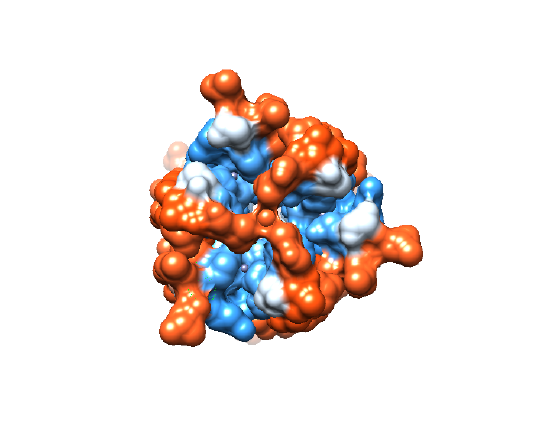
Interestingly, the ions flowing through the dermcidin channel do not travel in a straight path from the outer portion of the membrane to the inside of the bacterial cell. By looking at the the dermcidin channel from a bird’s eye view, Figure 6A shows the center through which ions travel, and Figure 6B shows the hydrophobicity of the channel’s opening. It is clear from Figure 6B that there are hydrophobic residues (red) blocking the entrance of ions directly through the pore termini. Because of this ‘capping’ of the channel that occurs, the transported ions need to utilize an alternative method to flow through the interior of the channel. Dermcidin accommodates for this problem by allowing the entry and exit of ions through the channel’s sides near the termini (Figure 7).
|
A. B. |
|
Figure 6. (A) An overhead view of the assembled dermcidin shows the pore through which ions are transported into the bacterial cell. (B) An overhead view of the assembled dermcidin with the electron density shows the ‘cap’ preventing ions from being transported through the open ends. (PDB: 2ymk) |
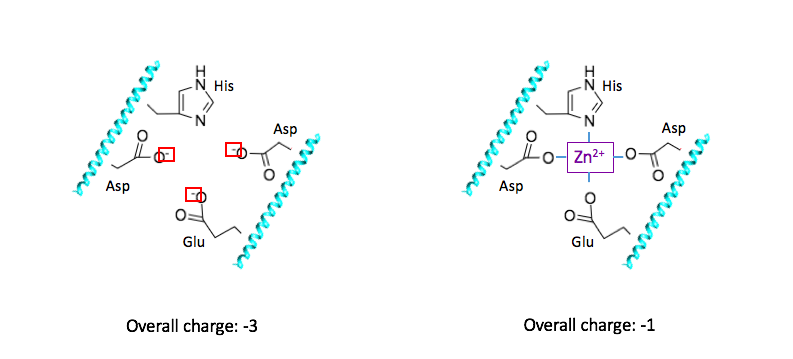
Why Zinc?
The metal center of dermcidin, which is zinc, has an oxidation state of 2+. The coordinated ligands, glutamate, and aspartate, are each negatively charged at physiological pH, giving a -3 charge for the complex before coordinating with Zn2+ (Figure 8A). When Zn2+ is coordinated, the overall charge of the coordination complex is decreased to -1(Figure 8B). The positive charge on the zinc helps the overall charge of the coordination complex become less negative. This increase in charge is beneficial to the function of dermcidin, as it can more effectively target the negatively charged bacterial membranes.
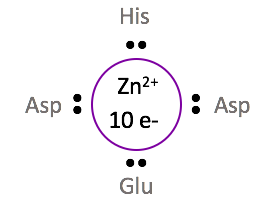
The +2 oxidation state of zinc gives Zn2+ a d-electron count of 10. The metal d-electron count is the number of electrons that are present in the largest d orbital. For the zinc metal ion coordinated in dermcidin, the +2 charge indicates that 2 electrons are absent from the 4s orbital, leaving the 3d orbital full with 10 electrons. With each ligand donating two electrons to Zn2+, and 10 electrons contributed from the d-orbital, the total electron count for this system is 18. The 18 d-electron count indicates that Zn2+ is coordinately saturated, and thus in its most stable conformation. For a metal ion to be coordinately saturated with four ligands, a d10 metal such as Zn2+ must be selected.
Coordination Geometry: Why Tetrahedral?
Due to the coordinated Zn2+ metal ion’s full d-orbital, its ligand field stabilization energy (LFSE) is zero (see below). A consequence of an LFSE of zero is a lack of preference toward a particular geometry.
LFSE = (-0.4x + 0.6y)ΔO
= (-0.4ᐧ6 + 0.6ᐧ4)ΔO
= 0ΔO
Calculation of the ligand field stabilization energy (LFSE) for Zn2+ where x is the number of electrons in the eg d-orbital, and y is the number of electrons in the t2g d-orbital.
As previously discussed, Zn2+ is coordinately saturated with four ligands, so the choice in geometry narrows down to either tetrahedral or square planar. By looking at the steric constraints and d-orbital splitting diagrams of each geometry, a geometry for this metal complex can be selected. Steric constraints are caused by the repulsion between ligands, which results in the ligands positioning themselves as far away as possible from its neighboring ligands.5 In a tetrahedral geometry, the bond angles between ligands are 109.5°, and in a square planar geometry, the bond angles between ligands are 90°.6 Based on these bond angles, the more sterically favorable geometry is tetrahedral.
Geometric constraints are an additional factor that could be determining the geometry of the metal complex in assembled dermcidin. Geometric constraints are caused by the limit in accessible conformations and the existing bond angles of the ligands.5 Due to the placement of the four coordinating ligands on their respective alpha helices, it is possible that the metal complex has no choice other than to have a tetrahedral geometry (refer to Figure 3B).
Thermodynamics of Zn2+ Binding to Dermcidin
In dermcidin, each Zn2+ ion is bound to four donor groups (Glu, His, Asp, Asp) at the termini of the channel structure (Figure 10). Two of the donor groups (Asp and His) come from one alpha helix, and the other two donor groups (Asp and Glu) come from an adjacent antiparallel alpha helix. Each of these helices is acting as a bidentate ligand, possessing two donor groups to which the zinc binds. The helix ligands each can bind to the Zn2+ at two donor group sites. Thus, each helix ligand acts as a chelator, which is a ligand that binds to a metal ion through more than one donor group.5 When the Zn2+ is bound to the four donor groups of the antiparallel helix pairs, the entropic penalty is decreased because each of the ligands possessing two donor groups is a chelator. The favorable reaction of binding to a chelator, is called the chelate effect. In order to understand how it is entropically favorable for zinc to bind to its chelators, it can be assumed that the Zn2+ is attached to water molecules prior to binding to the ligand donor groups. Thus, when the metal complex is formed, there is an increase in entropy as the number of product components is greater than the number of reactant components. This increase in entropy, in turn, increases the affinity of dermcidin for Zn2+ due to the chelate effect.
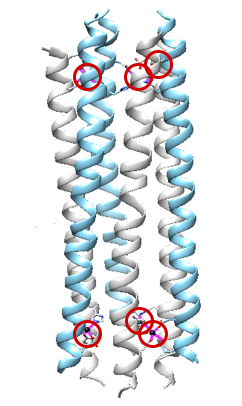
Dermcidin’s selectivity for Zn2+ can also be attributed to the types of metal-ligand interactions that are occurring. According to hard-soft acid-base theory, zinc is classified as a “borderline” acid and will prefer binding to borderline ligand donor groups (borderline bases). The histidine imidazole is classified as a “borderline” base, while both Asp and Glu are classified as “hard” bases. Due to the “mixed” hard/soft character of the ligands, the selectivity of a borderline metal, such as zinc, is reasonable.
The coordinated Zn2+ in dermcidin is a d10 metal, indicating an LFSE of zero, as stated previously. By merely looking at the lack of stabilization energy, the metal complex would be thermodynamically unstable, and consequently kinetically labile. Lability refers to how quickly a metal-ligand bond is broken, therefore a labile metal complex is able to bind and release from its coordinated metal ion in a flexible manner.5 The Zn2+ metal ion does have labile qualities due to its LFSE; however, its +2 positive charge allows strong electrostatic attraction with the negatively charged ligands. The stronger affinity between Zn2+ and its ligands result in the metal complex having more inert qualities compared to Zn2+ coordinating to neutral ligands. The relatively inert nature of the metal complex makes the metal-ligand bonds more difficult to break, and thus allows the dermcidin channel to remain structurally stable while it performs its antimicrobial function.
Sources
- Rieg, S.; Steffen, H.; Seeber, S.; Humeny, A.; Kalbacher, H.; Dietz, K.; Garbe, C.; Schittek, B. Deficiency of Dermcidin-Derived Antimicrobial Peptides in Sweat of Patients with Atopic Dermatitis Correlates with an Impaired Innate Defense of Human Skin In Vivo. The Journal of Immunology 2005, 174 (12), 8003–8010.
- Nguyen, V. S.; Tan, K. W.; Ramesh, K.; Chew, F. T.; Mok, Y. K. Structural Basis for the Bacterial Membrane Insertion of Dermcidin Peptide, DCD-1L. Sci Rep 2017, 7.
- Song, C.; Weichbrodt, C.; Salnikov, E. S.; Dynowski, M.; Forsberg, B. O.; Bechinger, B.; Steinem, C.; de Groot, B. L.; Zachariae, U.; Zeth, K. Crystal Structure and Functional Mechanism of a Human Antimicrobial Membrane Channel. Proc Natl Acad Sci U S A 2013, 110 (12), 4586–4591.
- Paulmann, M.; Arnold, T.; Linke, D.; Özdirekcan, S.; Kopp, A.; Gutsmann, T.; Kalbacher, H.; Wanke, I.; Schuenemann, V. J.; Habeck, M.; Bürck, J.; Ulrich, A. S.; Schittek, B. Structure-Activity Analysis of the Dermcidin-Derived Peptide DCD-1L, an Anionic Antimicrobial Peptide Present in Human Sweat. J Biol Chem 2012, 287 (11), 8434–8443.
- Adapted by K.Haas from Johnson, B. J.; Graham, K. J., A Guided Inquiry Activity for Teaching Ligand Field Theory. J Chem Educ 2015, 92 (8), 1369-1372.
- Geometry of Molecules https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/Lewis_Theory_of_Bonding/Geometry_of_Molecules (accessed Apr 14, 2018).
- Structural Motif. Wikipedia; 2017.
- Peters, B. M.; Shirtliff, M. E.; Jabra-Rizk, M. A. Antimicrobial Peptides: Primeval Molecules or Future Drugs? PLOS Pathogens 2010, 6 (10), e1001067.
- Goodsell, D. S. Dermcidin. RCSB Protein Data Bank 2013.
- Cell Envelope. Wikipedia; 2017.
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J. J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6 (11), 1887–1898.
Contributed By
This work was originally written by Sheila Lawler, Spring 2018: Sheila is currently (as of 2018) a junior chemistry major at Saint Mary's College in Notre Dame, IN. This work was originally edited by Madison Sendzik, Teaching and Research Assistant of Saint Mary's College.
Curated or created by Kathryn Haas