2.3: Hemiacetals, Hemiketals, and Hydrates
- Page ID
- 291128
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Overview
One of the most important examples of a nucleophilic addition reaction in biochemistry, and in carbohydrate chemistry in particular, is the addition of an alcohol to a ketone or aldehyde. When an alcohol adds to an aldehyde, the result is called a hemiacetal; when an alcohol adds to a ketone the resulting product is a hemiketal.
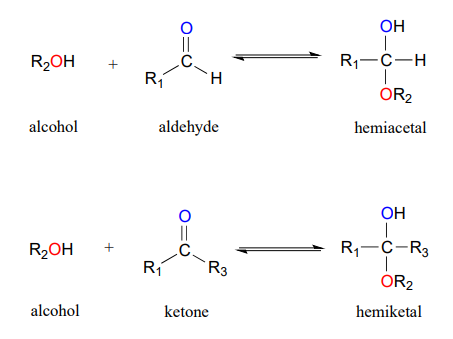
(The prefix ‘hemi’ (half) is used in each term because, as we shall soon see, addition of a second alcohol nucleophile can occur, resulting in species called acetals and ketals.)
The conversion of an alcohol and aldehyde (or ketone) to a hemiacetal (or hemiketal) is a reversible process. The generalized mechanism for the process at physiological pH is shown below.
Biochemical mechanism of hemiacetal formation:
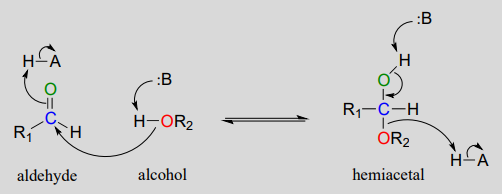
In general, hemiacetals (and hemiketals) are higher in energy than their aldehyde-alcohol components, so the equilibrium for the reaction lies to the left. As we will soon see in the context of glucose and other sugars, however, five- and six-membered cyclic hemiacetals are considerably lower in energy, and are favored at equilibrium: recall from last semester the inherent stability of five- and six-membered rings.
Aldehydes and ketones, when in aqueous solution, exist in equilibrium with their hydrate form. A hydrate forms as the result of a water molecule adding to the carbonyl carbon of the aldehyde or ketone.
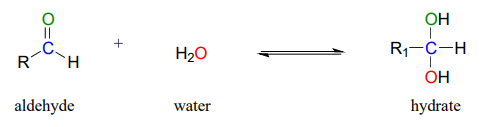
Although you should be aware that aldehyde and ketone groups may exist to a considerable extent in their hydrated forms when in aqueous solution (depending upon their structure), they are usually drawn in their non-hydrated form for the sake of simplicity.
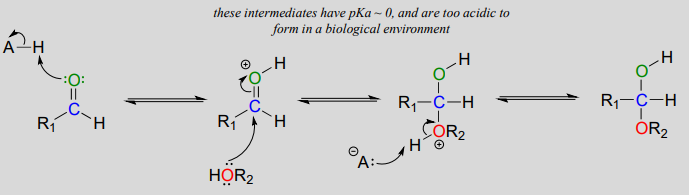
The mechanism we just saw for hemiacetal formation applies to biochemical reactions occurring at physiological \(pH\). In the organic laboratory, however, hemiacetal and hemiketal formation usually takes place in the presence of a strong acid. The acid catalyzes the reaction by protonating the carbonyl oxygen, thus increasing the electrophilicity of the carbonyl carbon. Notice in the mechanism below that highly acidic intermediates are drawn which would be unreasonable to propose for the corresponding biochemical mechanisms occurring at physiological pH.
Acid-catalyzed hemiacetal formation (non-biological):
Sugars as intramolecular hemiacetals and hemiketals
As stated above, the reactions of hemiacetals and hemiketals are central to the chemistry of carbohydrates. Recall that sugar molecules generally contain either an aldehyde or a ketone functional group, in addition to multiple alcohol groups. Aldehyde sugars are often referred to as aldoses; ketone sugars as ketoses. For example, glucose is an aldose, and fructose is a ketose - their structures are drawn below in Fischer projection:
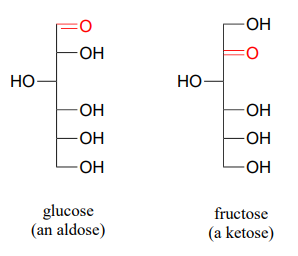
Exercise \(\PageIndex{1}\)
What term describes the relationship between glucose and fructose (in other words, what kind of isomers are they)?
Glucose and fructose are shown above in their open-chain form. However, in aqueous solution, glucose, fructose, and other sugars of five or six carbons rapidly interconvert between straight-chain and cyclic forms. This occurs through the formation of intramolecular hemiacetals and hemiketals. This simply means that the 'R' group of the alcohol is already covalently attached to the 'R 'group of the aldehyde (R1 in our general mechanism).
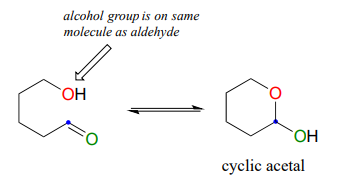
Unlike most of the biochemical reactions you will see in this text, sugar cyclization reactions are not catalyzed by enzymes: they occur spontaneously and reversibly in aqueous solution. For most five- and six-carbon sugars, the cyclic forms predominate in equilibrium.
The cyclic form of glucose is a six-membered ring, with an intramolecular hemiacetal formed by attack of the hydroxl on carbon #5 to the aldehyde carbon (carbon #1, also called the anomeric carbon in carbohydrate terminology).

The cyclic form of glucose is called glucopyranose. As was discussed above, nucleophilic attack on a planar carbonyl group can occur at either face of the plane, leading to two different stereochemical outcomes - in this case, to two different diastereomers. In carbohydrate nomenclature, these two diastereomers are referred to as the α and β anomers of glucopyranose.
Because the formation of glucopyranose occurs spontaneously without enzyme catalysis, shouldn’t equal amounts of these two anomers form? In fact, this does not happen: there is almost twice as much of one anomer than the other at equilibrium. Why is this? Remember (section 3.2) that six-membered rings exist predominantly in the chair conformation, and that the lower energy chair conformation is that in which unfavorable interactions between substituents are minimized – in most cases, this is the conformation in which larger substituents are in the equatorial position. In the lower-energy chair conformation of the major β anomer of glucopyranose, all of the hydroxyl groups are in the equatorial position, but in the minor a anomer one hydroxyl group is forced into the axial position. As a result, the α anomer is higher in energy, and less abundant at equilibrium.

Exercise \(\PageIndex{2}\)
Draw a mechanism for the conversion of α-glucopyranose to open-chain glucose.
Fructose in aqueous solution forms a six-membered cyclic hemiketal called fructopyranose when the hydroxyl oxygen on carbon #6 attacks the ketone carbon (carbon #2, the anomeric carbon in fructose).
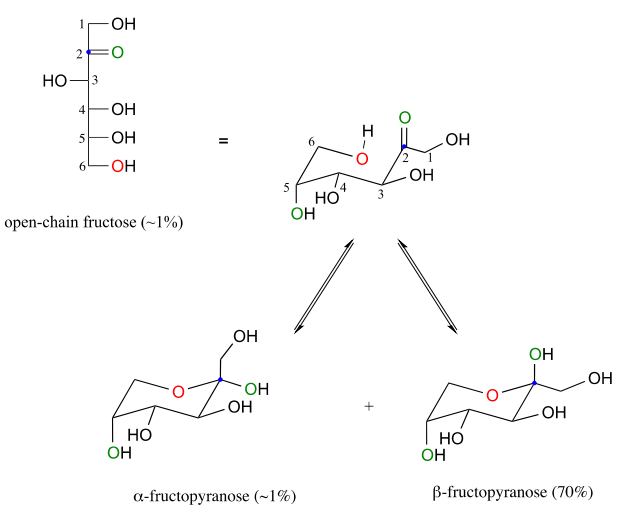
In this case, the β anomer is heavily favored in equilibrium by a ratio of 70:1, because in the minor a anomer the bulkier CH2OH group occupies an axial position.
Notice in the above figure that the percentages of α and β anomers present at equilibrium do not add up to 100%. Fructose also exists in solution as a five-membered cyclic hemiketal, referred to in carbohydrate nomenclature as fructofuranose. In the formation of fructofuranose from open-chain fructose, the hydroxyl group on the fifth carbon attacks the ketone.

In aqueous solution, then, fructose exists as an equilibrium mixture of 70% β-fructopyranose, 23% β-fructofuranose, and smaller percentages of the open chain and cyclic α-anomers. The β-pyranose form of fructose is one of the sweetest compounds known, and is the main component of high-fructose corn syrup. The β-furanose form is much less sweet.
Although we have been looking at specific examples for glucose and fructose, other five- and six-carbon monosaccharides also exist in solution as equilibrium mixtures of open chains and cyclic hemiacetals and hemiketals. Shorter monosaccharides are unlikely to undergo analogous ring-forming reactions, however, due to the inherent instability of three and four-membered rings.
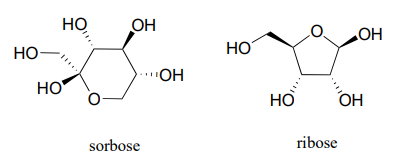
Exercise \(\PageIndex{3}\)
- Identify the anomeric carbon of each of the sugars shown below, and specify whether the structure shown is a hemiacetal or hemiketal.
- Draw mechanisms for cyclization of the open-chain forms to the cyclic forms shown.
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


