2.2: Nucleophilic Additions to Aldehydes and Ketones - An Overview
- Page ID
- 291127
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The aldehyde and ketone functional groups
Recall from before that the ketone functional group is made up of a carbonyl bonded to two carbons, while in an aldehyde one (or both) of the neighboring atoms is a hydrogen.
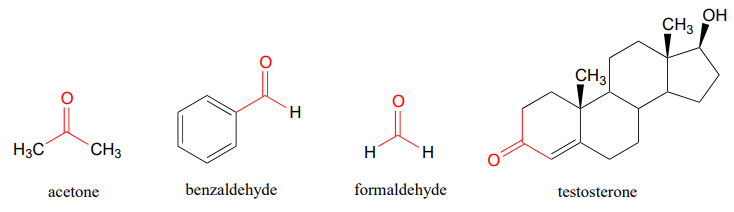
You probably are familiar with the examples shown below: acetone, the simplest ketone compound, is a common solvent in nail polish remover, benzaldehyde is the flavoring in maraschino cherries, and formaldehyde (a special case in which the carbonyl carbon is bonded to hydrogens on both sides) is the nasty-smelling stuff that was used to preserve the unlucky frog that you dissected in high school biology class. The male sex hormone testosterone contains a ketone group in addition to alcohol and alkene groups.
Recall from chapter 2 (last semester) the bonding picture in a ketone or aldehyde: the carbonyl carbon is sp2-hybridized, with its three trigonal planar sp2 orbitals forming σ bonds with orbitals on the oxygen and on the two carbon or hydrogen atoms. The remaining unhybridized 2p orbital is perpendicular to the plane formed by the sp2 orbitals, and forms a π bond through a side-by-side overlap with a 2p orbital on the oxygen. The σ and π bonds between the carbon and oxygen combine to make the C=O double bond that defines the carbonyl functionality.

Nucleophilic addition
The carbon-oxygen double bond is polar: oxygen is more electronegative than carbon, so electron density is higher on the oxygen end of the bond and lower on the carbon end. Recall that bond polarity can be depicted with a dipole arrow (A in the figure below), or by showing the oxygen as bearing a partial negative charge and the carbonyl carbon a partial positive charge (B).

A third way to illustrate the carbon-oxygen dipole (C in the figure above) is to consider the two main resonance contributors: the major form, which is what you typically see drawn in Lewis structures, and a minor but very important contributor in which both electrons in the p bond are localized on the oxygen, giving it a full negative charge. The latter depiction shows the carbon with an empty 2p orbital and a full positive charge.
However the bond polarity is depicted, the end result is that the carbonyl carbon is electron-poor – in other words, it is an electrophile. In addition, the trigonal planar geometry means that the carbonyl group is unhindered). Thus, it is an excellent target for attack by an electron-rich nucleophilic group, a mechanistic step called nucleophilic addition:
Nucleophilic addition to an aldehyde or ketone (enzymatic)
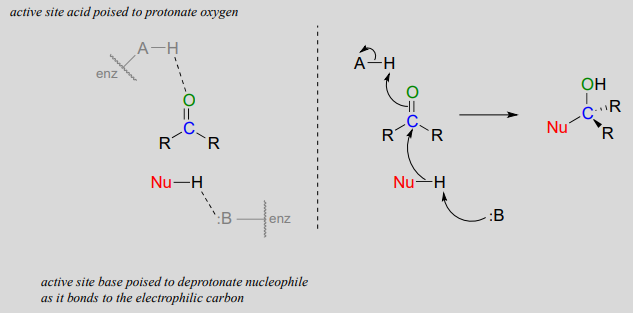
Notice the acid-base catalysis in this generalized mechanism: in the enzyme active site, a basic group is poised to deprotonate the nucleophile (thus enhancing its nucleophilicity) as begins to attack the carbonyl carbon, while at the same time an acidic proton on another active site group is poised just above the carbonyl oxygen (thus enhancing the electrophilicity of the carbon), ready to protonate the oxygen and neutralize any negative charge that builds up.
Stereochemistry of nucleophilic addition to a carbonyl
Recall from last semester that when the two groups adjacent to a carbonyl are not the same, we can distinguish between the re and si 'faces' of the planar structure.
The concept of a trigonal planar group having two distinct faces comes into play when we consider the stereochemical outcome of a nucleophilic addition reaction. Notice that in the course of a carbonyl addition reaction, the hybridization of the carbonyl carbon changes from sp2 to sp3, meaning that the bond geometry changes from trigonal planar to tetrahedral. If the two R groups are not equivalent, then a chiral center is created upon addition of the nucleophile. The configuration of the new chiral center depends upon which side of the carbonyl plane the nucleophile attacks from.
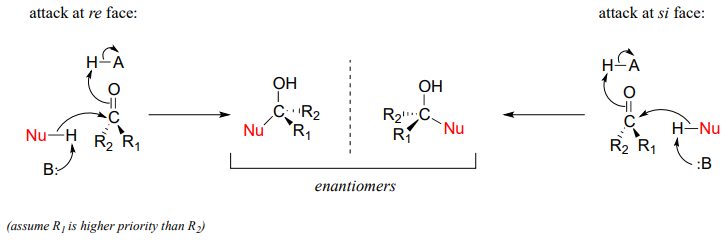
If the reaction is catalyzed by an enzyme, the stereochemistry of addition is (as you would expect) tightly controlled, and leads to one stereoisomer exclusively–the nucleophilic and electrophilic substrates are bound in specific positions within the active site, so that attack must occur specifically from one side and not the other. Nonenzymatic reactions of this type often result in a 50:50 mixture of stereoisomers, but it is also possible that one stereoisomer may be more abundant, depending on the structure of the reactants and the conditions under which the reaction takes place. We'll see some examples of this phenomenon soon when we look at cyclic forms of sugar molecules.
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


