2.4: Acetals and Ketals
- Page ID
- 291129
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Overview
Hemiacetals and hemiketals can react with a second alcohol nucleophile to form an acetal or ketal. The second alcohol may be the same as the first (ie. if \(R_2 = R_3\) in the scheme below), or different.
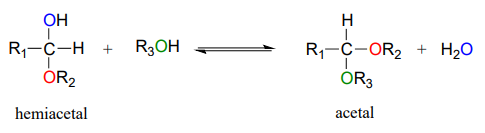
Although we focus here on biological reactions, it is instructive in this case to consider non-biological acetal-forming reactions before we look at their biochemical counterparts. In a non-enzymatic context, acetal/ketal formation - just like hemiacetal/hemiketal formation - is generally catalyzed by a strong acid.
Acid-catalyzed acetal formation (non-biological)
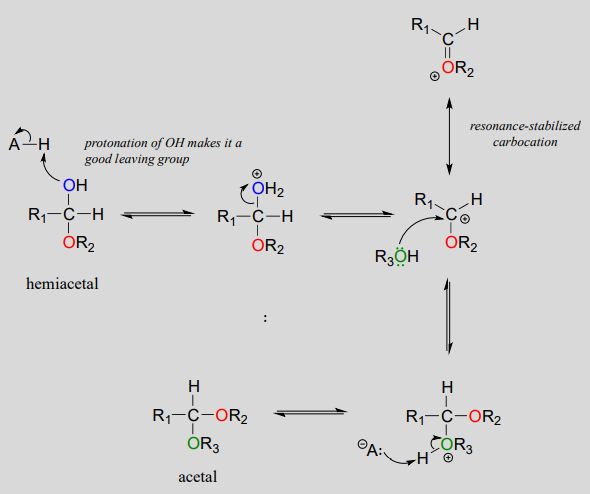
The role of the acid catalyst is to protonate the OH group of the acetal, thus making it a good leaving group (water). Notice something important here: the conversion of a hemiacetal to an acetal is simply an \(S_N1\) reaction, with an alcohol nucleophile and water leaving group. The carbocation intermediate in this \(S_N1\) mechanism is stabilized by resonance due to the oxygen atom already bound to the electrophilic carbon.
Below are some examples of simple, non-biological acetal and ketals.
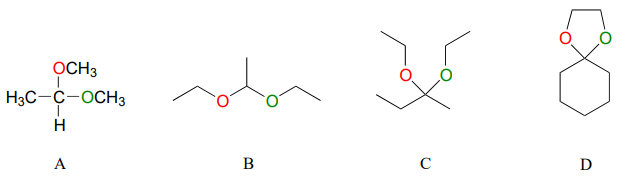
Exercise \(\PageIndex{1}\)
For each acetal/ketal A-D in the figure above, specify the required aldehyde/ketone and alcohol starting materials.
Exercise \(\PageIndex{2}\)
Categorize each of the following molecules as a hemiacetal, hemiketal, acetal, ketal, hydrate of an aldehyde, or hydrate of a ketone.
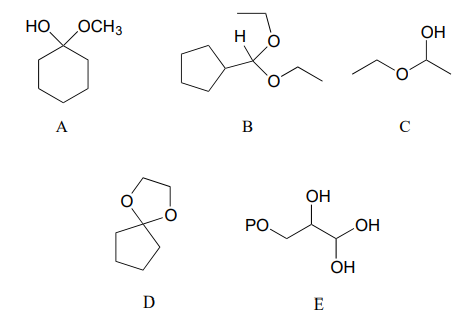
Exercise \(\PageIndex{3}\)
Specify the acetal/ketal that would form from a reaction between the given starting compounds.
a.
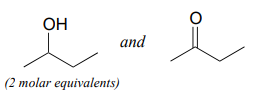
b.
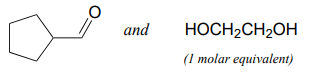
Exercise \(\PageIndex{4}\)
Specify the aldehyde/ketone and alcohol combination that would be required to form the compounds in exercise 10.5.
Glycosidic bond formation
Now, let's consider acetal formation in a biochemical context. A very important example of the acetal/ketal group in biochemistry is the glycosidic bonds which link individual sugar monomers to form polysaccharides (see section 1.3 for a quick review). Look at the glycosidic bond between two glucose monomers in a cellulose chain:
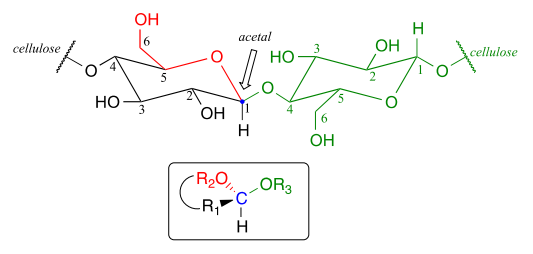
If you look carefully, you should recognize that carbon #1, the anomeric carbon on the left-side glucose monomer, is the central carbon of an acetal group. Biochemists refer to this as a β-1,4 linkage, because the stereochemistry at carbon #1 is β in the specialized carbohydrate nomenclature system, and it is linked to carbon #4 of the next glucose on the chain. The vast structural diversity of carbohydrates stems in large part from the different linkages that are possible - both in terms of which two carbons are linked, and also the stereochemistry of the linkage. You will see many more variations of glycosidic bond linkage patterns if you study carbohydrate biochemistry in greater depth.
Reactions in which new glycosidic bonds are formed are catalyzed by enzymes called glycosyltransferases, and in organic chemistry terms these reactions represent the conversion of a hemiacetal to an acetal (remember that sugar monomers in their cyclic form are hemiacetals and hemiketals). The mechanism for glycosidic bond formation in a living cell parallels the acid-catalyzed (non-biological) acetal-forming mechanism, with an important difference: rather than being protonated, the \(OH\) group of the hemiacetal is converted to a good leaving group by phosphorylation (this is a pattern that we are familiar with from chapter 9). The specific identity of the activating phosphate group varies for different reactions, so it is generalized in the figure below.
Mechanism for (biochemical) acetal formation:
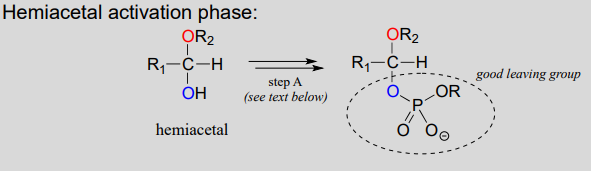
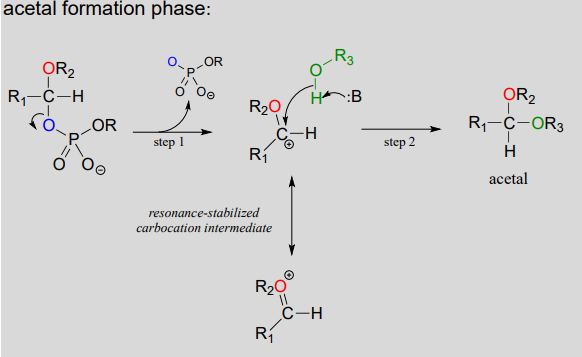
- Step A (Activation phase): This phase of the reaction varies according to the particular case, but always involves phosphate group transfer steps that are familiar from chapter 1. What is most important for our present discussion, however, is simply that the hydroxyl group on the hemiacetal has been activated (i.e., made into a good leaving group) by phosphorylation.
- Step 1: Now that the hydroxyl group has been activated by converting it to a good leaving group, it does its job and leaves, resulting in a resonance-stabilized carbocation.
- Step 2: A nucleophilic alcohol on the growing cellulose chain attacks the highly electrophilic carbocation to form an acetal. Here is where the stereochemistry of the new glycosidic bond is determined: depending on the reaction, the alcohol nucleophile could approach from either side of the planar carbocation.
To reiterate: it is important to recognize the familiar \(S_N1\) mechanistic pattern in play here: in step A, a poor leaving group is converted into a good leaving group, in step 1 the leaving group leaves and a stabilized carbocation is left behind, and in step 2 a nucleophile attacks to form a new bond and complete the substitution process. Look back at the \(S_N1\) reactions we saw last semester if you are having trouble making this mechanistic connection.
Now, let's look specifically at the glycosyl transferase reaction mechanism in which a new glycosidic bond is formed on a growing cellulose chain. Glucose (a hemiacetal) is first activated through two enzymatic phosphate transfer steps: step A1, a phosphate isomerization reaction with a mechanism similar to the reaction in problem P1.13, followed by a UTP-dependent step A2, for which you were invited to propose a mechanism in problem P1.12.
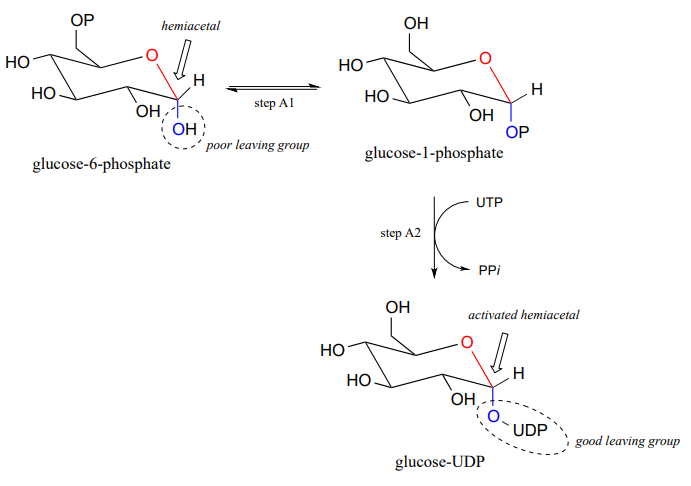
The UDP group on glucose-UDP then leaves (step 1 below), forming a resonance-stabilized carbocation intermediate. Attack by the alcohol group on the growing cellulose chain in step 2 forms the glycosidic (acetal) bond. Note the inversion of stereochemistry.
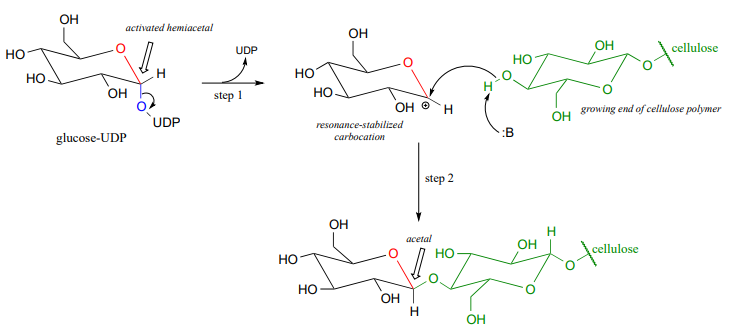
Glycosidic bond hydrolysis
Acetals can be hydrolyzed back to hemiacetals. Notice that an acetal to hemiacetal conversion is an \(S_N1\)-type reaction with a water nucleophile and an alcohol leaving group.
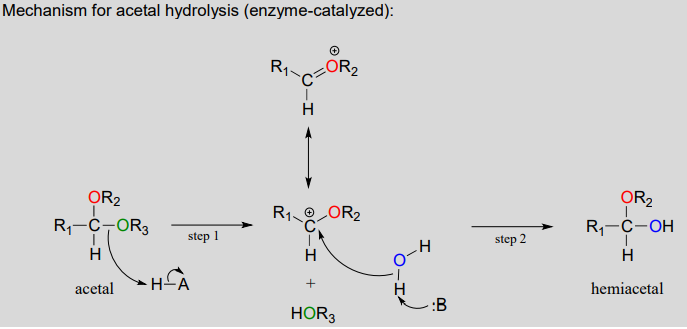
In step 1, an alcohol is protonated by a nearby acid group as it breaks away to form a resonance-stabilized carbocation intermediate. The carbocation is attacked by a nucleophilic water molecule in step 2 to form a hemiacetal.
The general mechanism above applies to reactions catalyzed by glycosidase enzymes, which catalyze the cleavage of glycosidic bonds in carbohydrates. In the introduction to this chapter, we learned about ongoing research in the field of cellulosic ethanol. Recall that the main bottleneck in the production of ethanol from sources such as switchgrass or wood is the cellulase-catalyzed step in which the glycosidic bonds in cellulose are cleaved. Cellulose-digesting microbes have several different but closely related forms of cellulase enzymes, all working in concert to cleave cellulose into smaller and smaller pieces until individual glucose molecules are free to be converted to ethanol by the fermentation process. Below is a representative mechanism for a cellulase reaction.
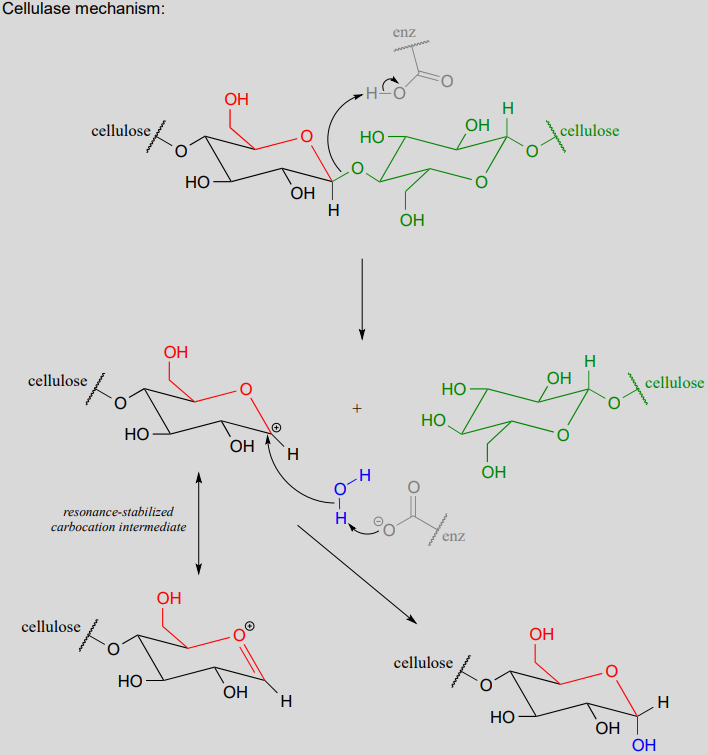
The starch-digesting amylase enzymes used in the corn ethanol production process catalyze similar glycoside hydrolysis reactions, the main difference being the opposite stereochemistry at the anomeric carbon of the substrate.
Exercise \(\PageIndex{5}\)
Notice that the cellulose glycososide bond-forming reaction requires the cell to 'spend' a high-energy UTP molecule, but the cellulase glycoside bond-breaking reaction does not. Use your knowledge of chemical thermodynamics to explain this observation.
Exercise \(\PageIndex{6}\)
Below is the structure of the artificial sweetener sucralose. Identify the two anomeric carbons in the disaccharide.
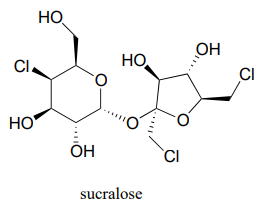
Exercise \(\PageIndex{7}\)
Robinose is a disaccharide found in 'Chenille Plant', a flowering shrub native to the Pacific Islands.
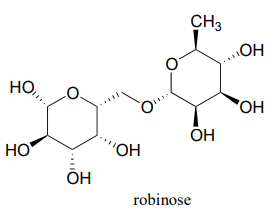
- Identify the two anomeric carbons and the glycosidic bond in robinose.
- Using the same carbon numbering system as for glucose in the earlier figure, fill in in the carbon numbers (#1 through #6) for each of the monosaccharides that make up robinose.
- Based on what you know of glycosidic bond-forming reactions in nature, propose a reasonable mechanism for the linking of the two monosaccharides, starting with the activated hemiacetal species, assuming that it is a UDP species as in the cellulose gycosidic bond-forming reaction.
- Draw the open chain form of each of the monosaccharides
Exercise \(\PageIndex{8}\)
Look again at the structures of the two-glucose fragments of cellulose and amylose shown the introduction to this chapter. A structural feature of the cellulose polymer makes it inherently more resistant to enzymatic hydrolysis compared to starch. Explain.
- Hint
-
Think about intermolecular interactions.
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


