7.2: Chirality
- Page ID
- 321429
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chirality is a subset of stereochemistry that deals with molecules that rotate plane-polarized light, which is different from stereoisomers that cannot do so, such as geometric isomers. A molecule is considered chiral if it can rotate plane-polarized light, and will always possess a nonsuperimposable mirror image. The most common examples of chiral molecules are those that contain a stereocenter (there are others that do not fall into this category – we will discuss these later).
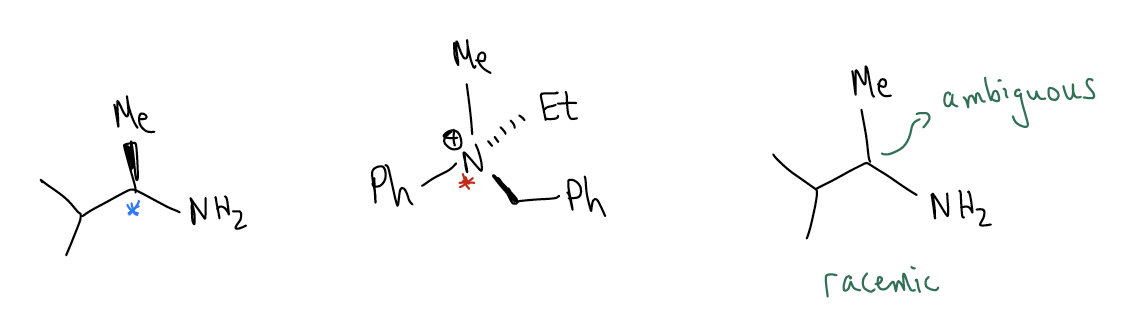
A stereogenic center (stereocenter, or chiral center) is a tetrahedral atom that contains four different groups attached. This means that the molecule itself has a nonsuperimposable mirror image, or enantiomer. For example, the molecule on the left below has a stereogenic carbon atom. Likewise, the molecule in the middle has a chiral nitrogen atom. The molecule on the right is the same as the molecule on the left, yet no stereochemistry has been provided to us, so we can assume that the chirality is ambiguous and it is a 1:1 mixture of enantiomers (racemic mixture).
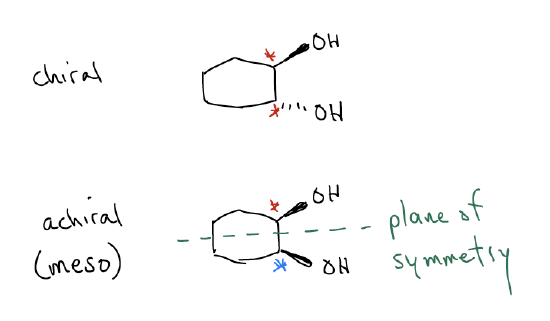
A molecule that contains a stereocenter can be considered chiral, but this is not always the case. Consider again the following two molecules. These molecules are diastereomers because they are nonsuperimposable non mirror image isomers, but only one of them is chiral. Each molecule contains two stereocenters, however, only the molecule on the left is chiral because it does not have an internal plane of symmetry. Stated another way, the internal plane of symmetry in the second molecule would cancel the rotation of plane-polarized light. The top half of the molecule would rotate the molecule in one direction while the bottom half of the molecule (since it is a mirror image) would rotate it the opposite way. Think of it like having a racemic mixture in the same molecule. The optical rotation is zero and we say that this molecule is achiral (not chiral). It has stereocenters, but the molecule as a whole will not rotate plane polarized light. These types of molecules, which have stereocenters that generate an internal plane of symmetry, are called meso compounds. Another example of a meso compound is a molecule that has an inversion center (center of symmetry). For example, a molecule can be achiral if when you rotate it 180° and then flip it, you get the same compound. It is as if the center of the molecule is a point of symmetry.
Chirality Beyond Carbon Stereocenters
Chirality exists outside the traditional description of four different groups attached to a tetrahedral carbon atom. Other chiral centers include P-, N-, and S-containing molecules, as shown below. Each of these has a nonsuperimposable mirror image, so they are chiral.
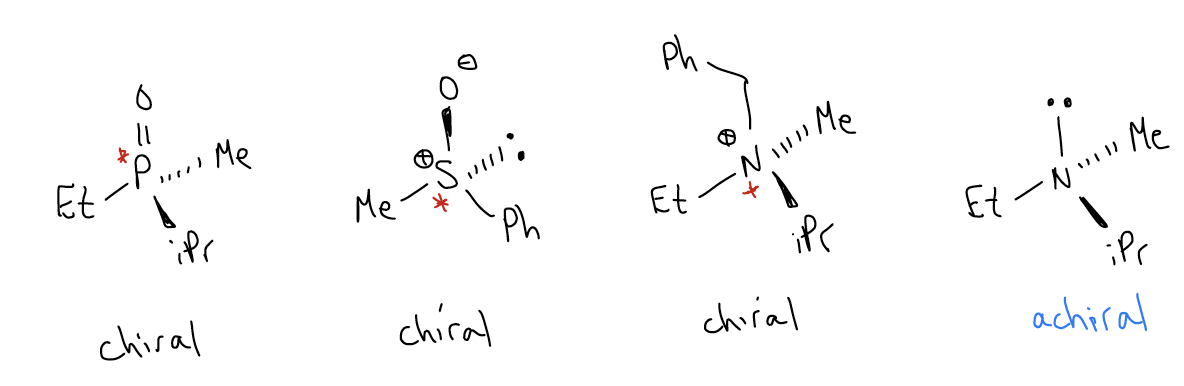
It should be noted that amines (containing three different groups AND a lone pair) are not chiral because at room temperature, the trigonal pyramidal geometry inverts rapidly, giving you a racemic mixture. However, phosphines (containing three different groups AND a lone pair) are chiral because they do not invert at room temperature. The barrier to pyramidalization is too high, so single enantiomers can be isolated.
Other molecules can be chiral even though they may not even contain a stereocenter. But they still satisfy the definition of having nonsuperimposable mirror images, so they are still chiral!
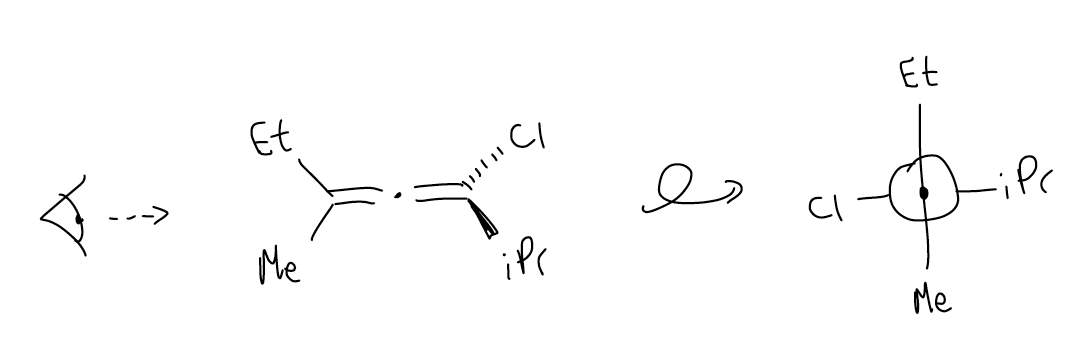
1. allenes have a form of chirality known as axial chirality. In these molecules, it is as if the central sp-hybridized carbon contains “four” different groups attached.
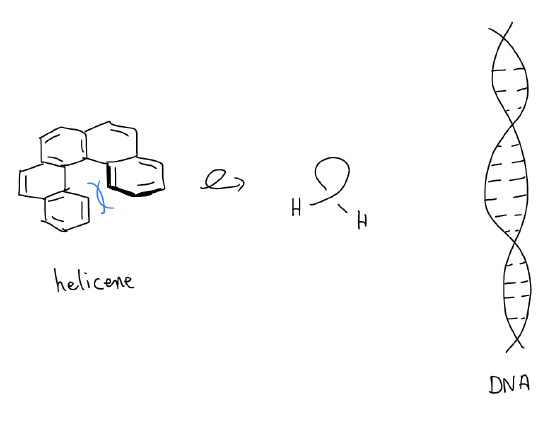
2. helicenes are hydrocarbons that twist. Similarly, the DNA double helix twists with a certain “handedness,” so it is also chiral.
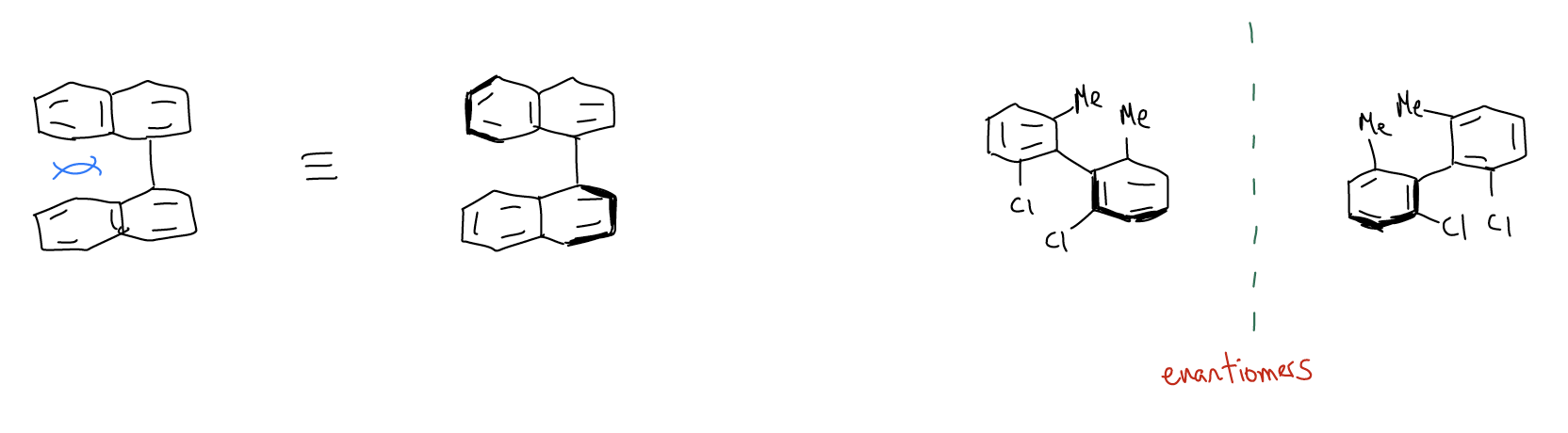
3. atropisomers occur when there is hindered rotation about a s-bond. This is another type of axial chirality.
Biomedical Spotlight
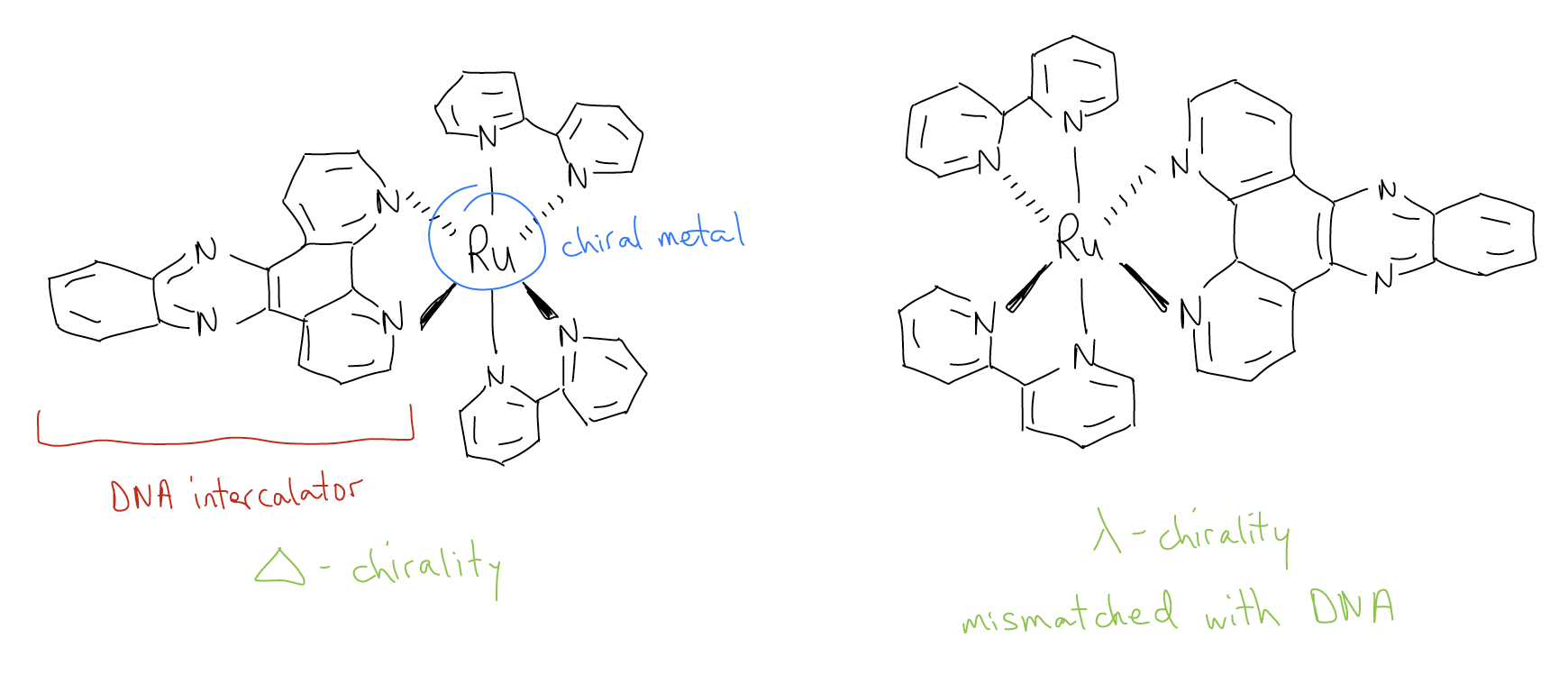
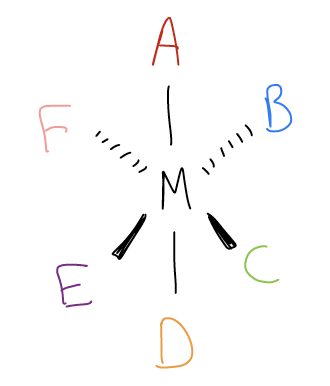
Transition metals also have the potential for chirality, but given their expanded coordination numbers, many more stereoisomers can result. Take, for example, an octahedral complex that contains six different monodentate ligands. One can arrange these ligands to give 32 unique stereoisomers!
This rich stereochemical diversity can be harnessed to selectively bind DNA. Consider the ruthenium-based polypyridyl complexes pioneered by Jacqueline Barton. These molecules contain three different bidentate ligands surrounding an octahedral ruthenium center. The planar nature of one of the ligands is capable of inserting into the base pairs of DNA and causing the DNA helix to unwind. Since DNA is chiral itself (a helicene with a right-handed twist!), only one of the two enantiomers will bind to DNA with sufficient strength. Convince yourself that the two molecules below are enantiomers of one another (that is, that they are non-superimposable mirror images of one another).