7.7: Resolution
- Page ID
- 321434
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Finally, we have said that enantiomers are equal in energy and have similar physical properties. One interesting question you might ask is: we are composed of chiral molecules, so how did chirality come into being in the first place. As in, evolutionarily? This is a large area of research, for which we do not have answers for, because we know that we cannot create chirality from achiral material. A similar, more practical consideration is: once we make a racemic mixture, how do we separate the enantiomers into enantiopure samples?
A resolution is the separation of a racemic mixture into its respective enantiomers. Since enantiomers are identical, they must first be converted into diastereomers, which have different energies and are easy to separate. The strategy here is to use acid-base chemistry to create diastereomeric salts that have differential solubility. One diastereomer will crystallize while the other will remain soluble. Simple filtration will provide a means of physical separation.
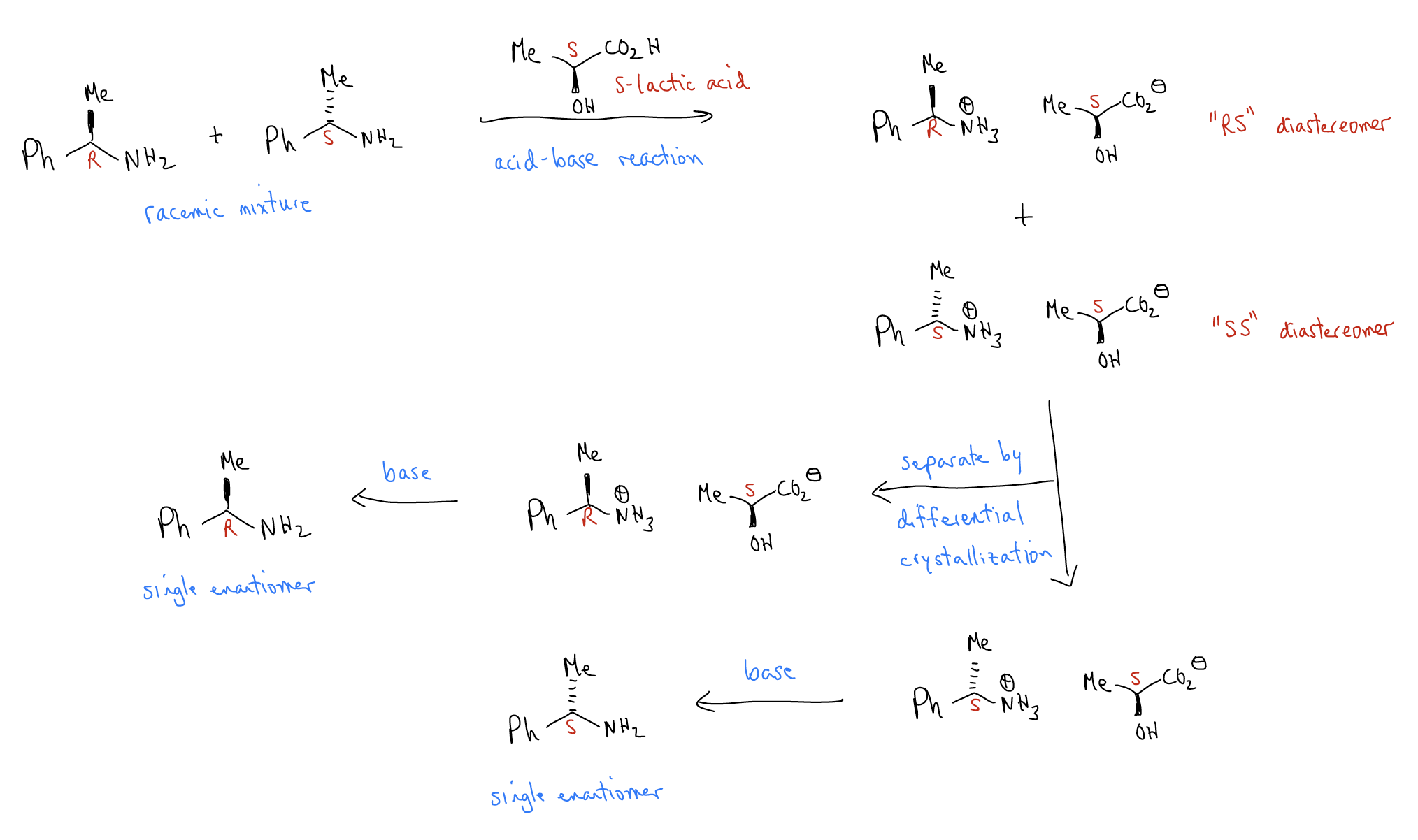
Other types of resolutions include enzymatic resolutions, where an enzyme preferentially binds one enantiomer and performs a chemical reaction on it, or chiral chromatography, where selective adhesion of one enantiomer to a chiral stationary phase allows separation of isomers.
Biomedical Spotlight
Living organisms have evolved to be mostly homochiral, meaning that the carbon stereocenters have a single, consistent stereochemistry. Because proteins, nucleic acids, and carbohydrates have a single handedness, it should come as no surprise that the interaction of these large macromolecules with the enantiomers of chiral small molecules are different. In essence, diastereomeric pairs of protein and racemic small molecule are created that should have different energies. This is important when considering the interaction of these drugs with macromolecules in live tissue. If one enantiomer binds tightly and the other one doesn't, would it not make more sense to develop a synthesis in which only a single enantiomer is created. Furthermore, what if one enantiomer had therapeutic effects and the other enantiomer was unsafe? In this case, would the development of a drug as a racemic mixture be ethical?
Believe it or not, these stereochemical considerations are more recent that one might imagine. The Food and Drug Administration (FDA), which governs the regulation of the pharmaceutical industry, only issued guidelines regarding the stereochemistry of drug-like molecules in 1992. These guidelines require that any drug that is chiral must be developed as a single enantiomer with defined absolute configuration and that the stereochemistry of the drug be monitored for degradation, metabolism, or even racemization/interconversion. If a drug is prepared and marketed as a racemic mixture, enough testing is required to demonstrate that the individual enantiomers do not cause any harm. As a result of these guidelines, most drugs are developed as single enantiomers from the beginning.
One famous (and sad) example of the need for more regulation in drug development is the story of thalidomide. Thalidomide is on the "List of Essential Medicines" that is compiled the World Health Organization. It was primarily prescribed as a treatment for morning sickness in the late 1950's, but has recently been used to treat multiple myeloma and leprosy. When it first became popular in West Germany to treat nausea in pregnant women, it was marketed and sold as a racemic mixture. While the R-enantiomer is the bioactive form, the S-enantiomer turned out to be teratogenic, meaning it resulted in severe birth defects including limb deformities. Furthermore, it was discovered that the the two enantiomers readily interconvert under biological conditions due to the highly acidic proton adjacent to the carbonyl, so development of a single enantiomer drug was not viable. As a result, physicians prescribe this drug with extreme caution, and only to those patients who are not pregnant or plan to become pregnant.
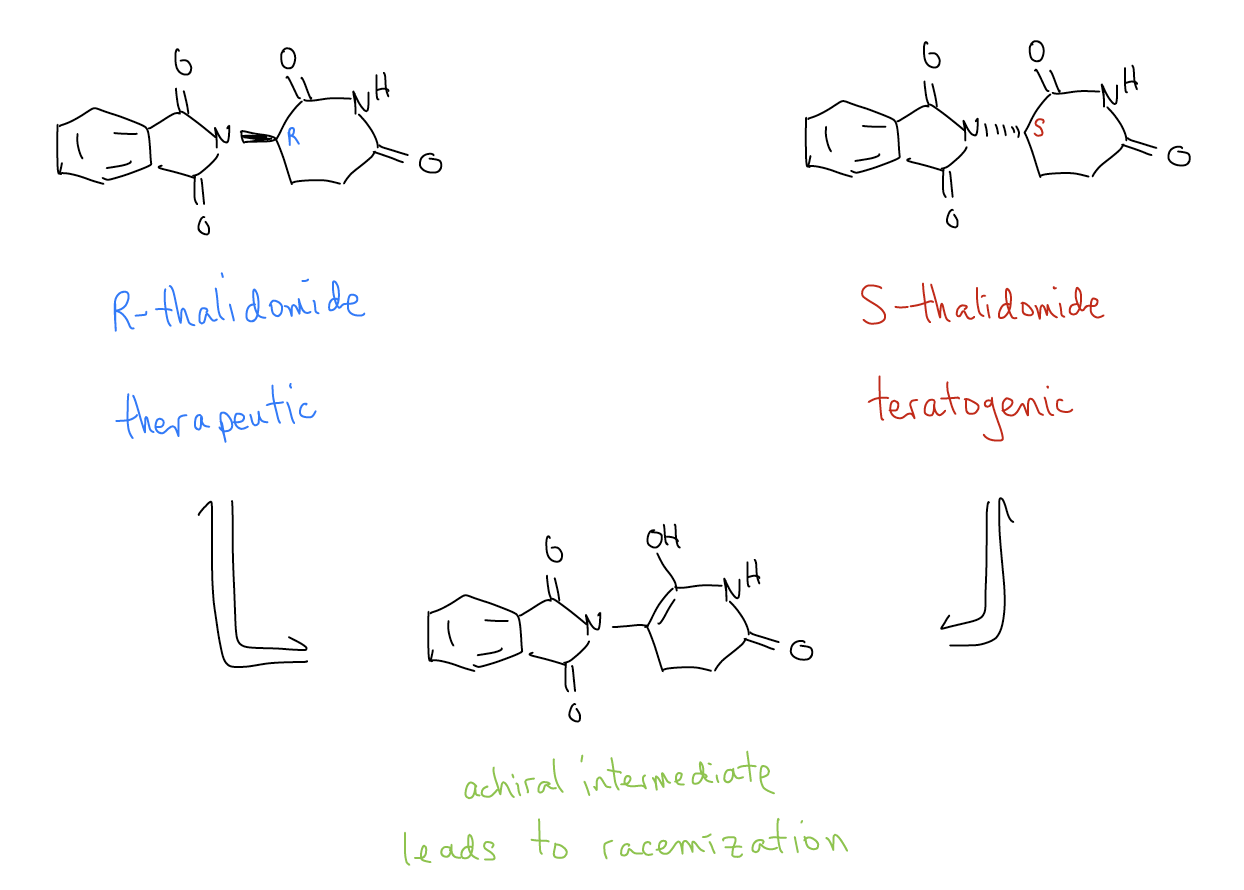
While tragic, this story was one of the reasons for the passage of the Kefauver-Harris Drug Amendments Act of 1962 that required drug makers to demonstrate the safety and efficacy of the drugs they manufacture. Thalidomide was further made famous by the song "We Didn't Start the Fire," by Billy Joel, who sang about the "children of thalidomide" in 1989.

