4.5: Fused Ring Systems
- Page ID
- 319867
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
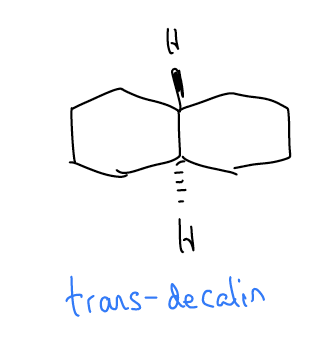
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have already discussed isolated ring systems, but what about rings that are fused with other rings – how might their conformations change? Well, nothing really changes except that the rings are fused. Fused rings still adopt the most stable ring conformations. In the case of six-membered rings, this would be the chair conformation. Consider the molecule trans-decalin, which is a set of fused six-membered rings where the hydrogen atoms at the ring fusion carbons are trans.
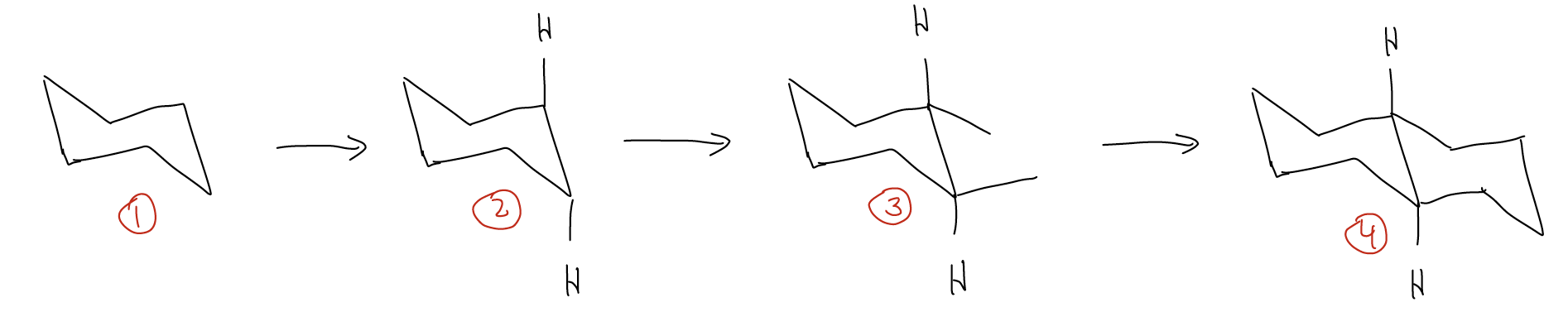
- Start by drawing a chair conformation for one ring
- Place the substituents on the ring fusion carbons, according to their stereochemistry depicted in the 2-D drawing
- Finish the rest of the conformation
What about rings that are cis-fused? We follow the same set of steps. Consider cis-decalin: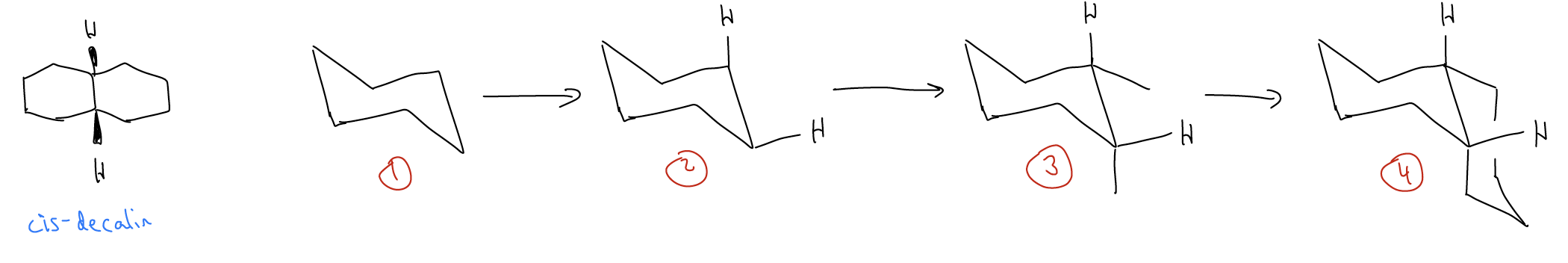
Quick question: which of these two rings, cis- or trans-decalin is more stable? Trans-decalin, of course, because it places substituents farther away from each other. There are no gauche interactions in the trans-decalin.
How would you perform a ring flip for a cis-decalin? We would need to do this in order figure out which conformation is most stable Let’s take one example, shown below. This ring flip is hard to visualize, so build a model, do the ring flip, and then redraw what you see.
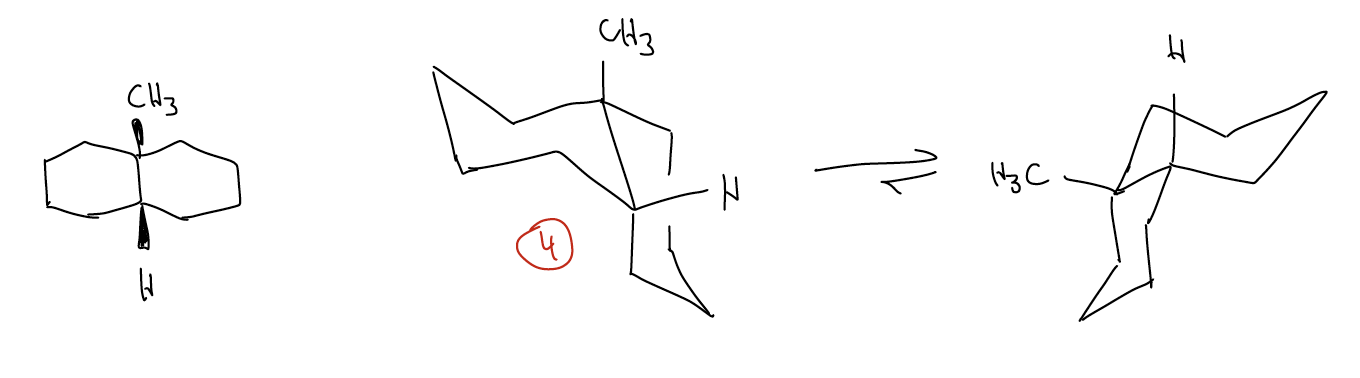
What about a ring flip for a trans-decalin? It can’t be done - this conformation is locked! You can't move the two equatorial bonds axial, nor can you move the two axial substituents equatorial - this is a constraint of the system itself.
Putting all of this together means that we can draw conformations of complex polycyclic systems. Consider the first molecule you came across this semester, betulin:
- Start with one of the 6-membered rings and work outward
- Connect the rings in a systematic fashion as before!
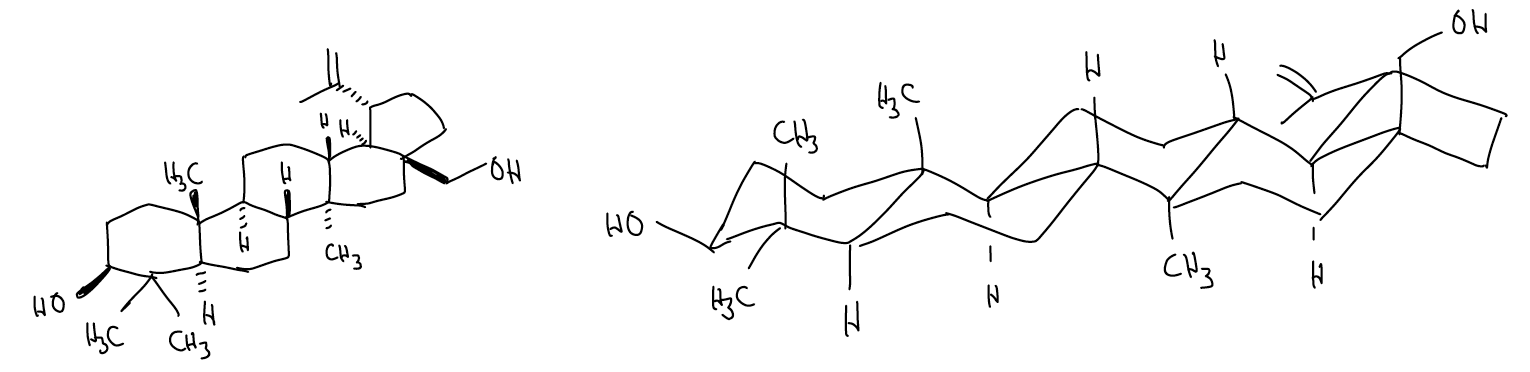
Biomedical Spotlight
Steroids are polycyclic, fused hydrocarbons that play an important role in biological processes. The main sex hormones - estrogen and testosterone, for example - have similar structures that play a huge role in the development of male and female characteristics throughout the life cycle. Another important steroid is cholesterol, which is essential for health and well-being. Cholesterol is a major component of the phospholipid bilayer that give cells their structure and function, it is a precursors to the bile acids that aid in the digestion of fats, and it gets converted into many of the steroids needed for cell signaling and regulation (including estrogen and testosterone). But, like most small molecules, too much of it can be a problem. Elevated blood levels of cholesterol are linked to atherosclerosis (hardening of the arteries) and increased risk of heart attack and stroke. We get cholesterol from our diet, but it is also made endogenously. Decades ago, chemists identified the biosynthetic pathway for cholesterol. More than 30 steps are needed to make cholesterol from acetic acid, all of which involve carefully regulated enzymatic activity. In the 1970s, Merck saw an opportunity to use this pathway to solve a growing health problem. Merck scientists hypothesized that cholesterol levels could be reduced to acceptable levels by inhibiting the activity of the protein hydroxymethylglutaryl-CoA reductase (HMGR). This enzyme converts HMG-CoA to mevalonic acid, but it is the slowest enzyme in the 30-step sequence. Years of work were needed to identify a small molecule that could inhibit HMGR in vitro, reduce cholesterol levels in clinical patients, and be safe for human consumption. The result were the statin drugs, of which Merck's lovastatin (Mevacor™) is a leading member. The statins are multi-billion dollar drugs that have had an tremendous impact on reducing high cholesterol in patients, thereby significantly reducing heart disease and stroke as leading causes of premature death.
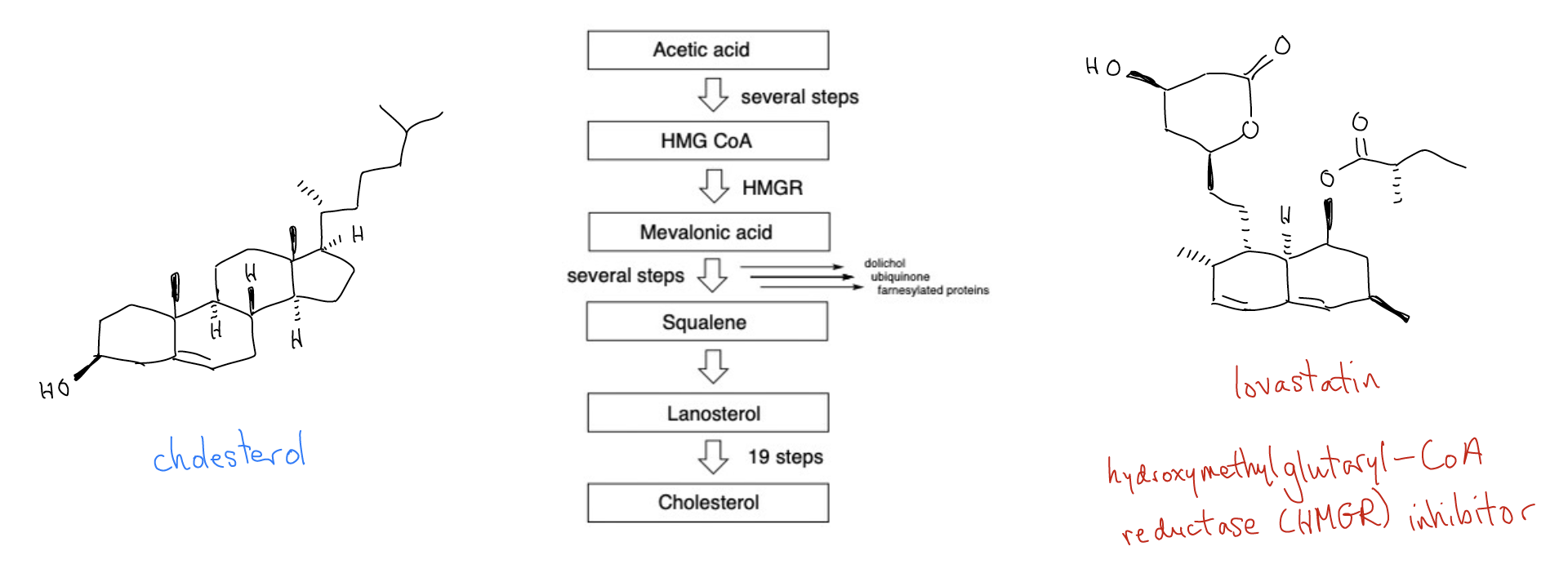
Adapted from Hallelujah Moments, by Eugene Cordes, 2014

