2.7.2: Pearson's Hard-Soft Acid-Base Concept
- Page ID
- 443640
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Hard-Soft Acid-Base concept is a tool for thinking about patterns of Lewis acid-base reactivity. Arland, Chatt, and Davies' observed that Lewis acids and bases could be classified into two groups based on their propensity to form stable compounds with one another (e.g. acids in a class tend to form more stable adducts with bases in the same class than they did with bases in the other class).1 Arland, Chatt, and Davies somewhat boringly termed these groups class a and class b but today they are known by Ralph Pearson's name for them. Pearson called the class a acids and bases hard and called the class b acids and bases soft. These terms reflect the polarizability of the electron cloud, or how "soft" these substance's electron clouds are towards distortion (Figure \(\PageIndex{1}\)). Soft acids and bases are relatively polarizable, and hard acids and bases are difficult to polarize.
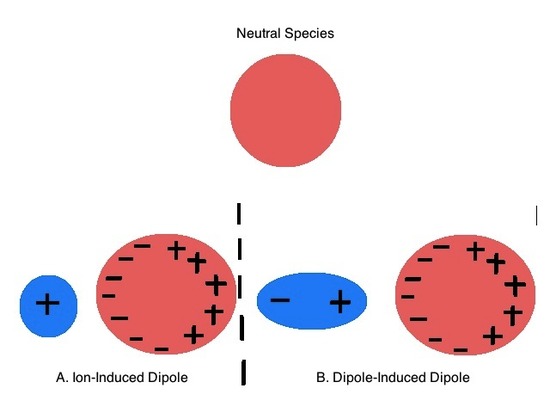
Hard and Soft Classification
Hard Species
Hard acids and bases have a high charge density (highly charged relative to size), and polarization is difficult. Low polarizability is attributed to hard acids being relatively electron-poor and hard bases being relatively electron-rich. Low polarizability is also attributed to the localization of charge on an atom with electron-withdrawing substituents (in the case of a hard acid) or electron-donating substituents (in the case of a hard base). It is also observed that hard acids have high oxidation states or large positive formal charges, while hard bases have low oxidation states or negative formal charges.
For hard acids, which possess few valence electrons, polarization involves distorting core (inner shell) electrons, which are difficult to distort because they are close to the nucleus and experience a high nuclear charge. Hard acids tend to be found towards the left side of the periodic table and involve higher oxidation states and/or electron donating substituents
Soft Species
Soft acids and bases posses many valence electrons and are more readily polarized. Soft acids and bases are more likely to be found towards the middle or right of the periodic table. Soft acids and bases also have little charge density and/or are relatively electron rich. Soft acids are more common to the right of the periodic table and involve lower oxidation states and/or electron donating substituents.
Borderline Species
All hard acids and bases are not equally hard, and all soft acids and bases are not equally soft. There is a graduation in hardness and softness, and there are a number of intermediate acids and bases that do not fit neatly in either category. Figure \(\PageIndex{1}\) classifies each element as a hard, soft, or borderline (intermediate) acid or base. Some borderline acids can be considered hard or soft depending on their oxidation state. The higher oxidation state species can be considered hard acids, and the lower oxidation state species can be considered soft acids. Additional representative examples of hard, soft, and borderline bases are shown in Figure \(\PageIndex2}\) and Figure \(\PageIndex{3}\).
 Figure \(\PageIndex{1}\): Pearson's Hard-Soft Acid Base Classification of the Elements (CC-BY-NC-SA; Kathryn A. Newton)
Figure \(\PageIndex{1}\): Pearson's Hard-Soft Acid Base Classification of the Elements (CC-BY-NC-SA; Kathryn A. Newton)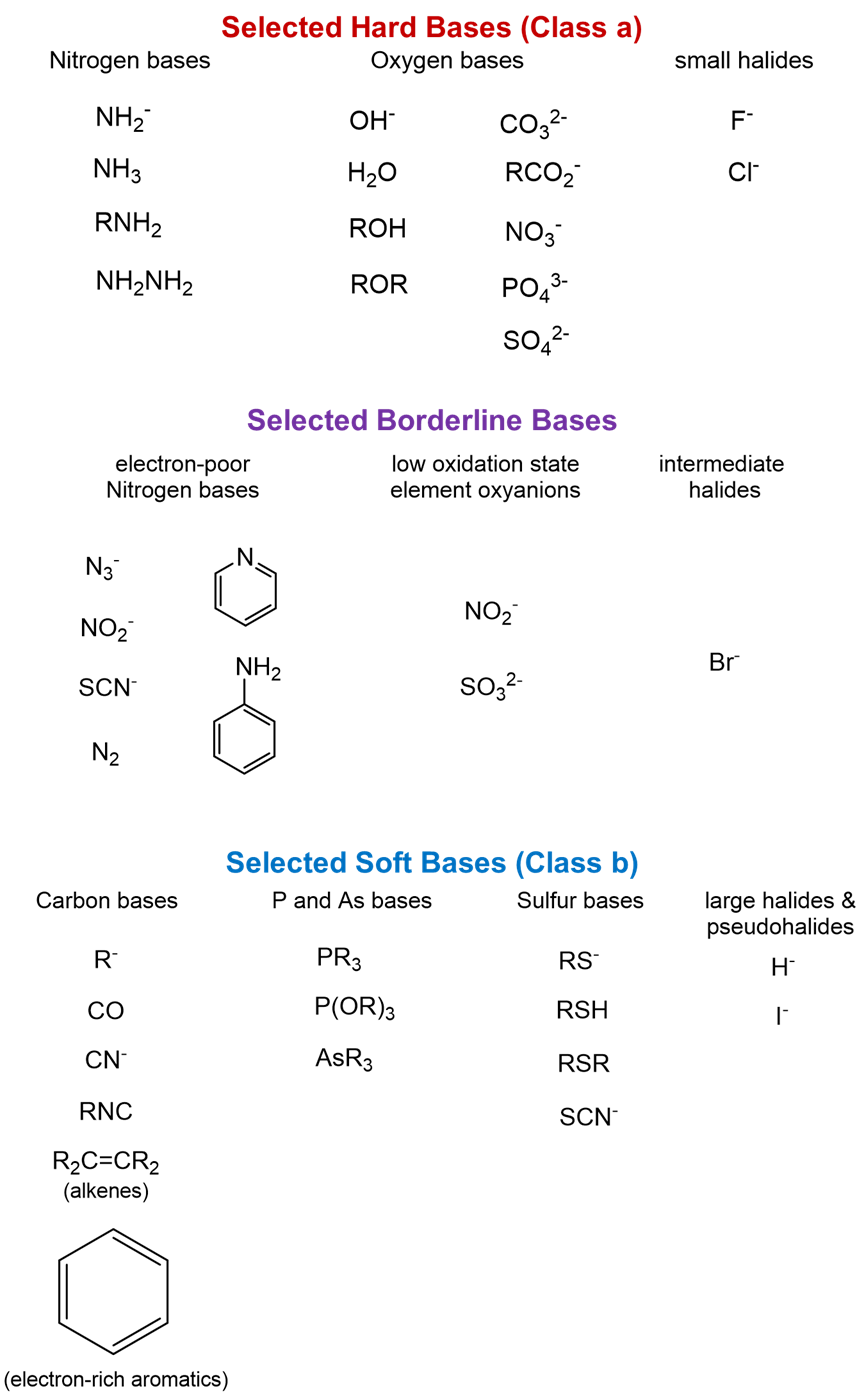
 Figure \(\PageIndex{3}\): HSAB classifcation of selected species. (CC-BY-NC-SA; Kathryn A. Newton)
Figure \(\PageIndex{3}\): HSAB classifcation of selected species. (CC-BY-NC-SA; Kathryn A. Newton)The Hard-Soft Acid-Base Principle (HSAB Principle)
The Hard-Soft Acid-Base Concept (HSAB Concept) explains patterns in Lewis acid-base reactivity as a like reacts with like preference. Both thermodynamically and kinetically, hard acids prefer hard bases and soft acids soft bases. Specifically,
- Thermodynamically, hard acids form more stable acid-base complexes with hard bases while soft acids form more stable complexes with soft bases.
- Kinetically, hard acids/electrophiles react more quickly with hard bases/nucleophiles while soft acids/electrophiles react more quickly with soft bases/nucleophiles.
The HSAB principle can predict the equilibrium of Lewis acid-base metathesis and displacement reactions. In a Lewis acid-base metathesis reaction the acids and bases swap partners:
\[\ce{A1:B1 + A2:B2 <=>[k_1, K_{eq}] A1:B2 + A2:B1} \nonumber \]
For example, the equilibrium position of the metathesis reaction between \(\ce{TlF}\) and \(\ce{K2S}\) favors the products:
\[\ce{2TlF + H2S <=>> Tl2S + 2KF} \nonumber \]
This is consistent with the HSAB's hard-hard and soft-soft preference.
 \[ \nonumber \]
\[ \nonumber \]
The HSAB principle also allows for prediction of displacement reactions, in which a Lewis acid or base forms an adduct using a base or acid from an existing Lewis acid-base complex. In these reactions, the displacement of acid or base from the reactant complex may be thought of as a sort of metathesis reaction, one in which in the unbound acid or base switches places with one in the complex. For example, the reaction between \(\ce{HI}\) and methylmercury cation involves displacement of an iodide from \(\ce{HI}\) to give \(\ce{CH3HgI}\). The position of the equilibrium favors \(\ce{CH3HgI}\) since both \(\ce{CH3Hg^{+}}\) and \(\ce{I^{-}}\) are soft, while \(\ce{H^{+}}\) is a hard acid.
\[\ce{HI + HgSCH3^{+} <=> CH3SHgI + H^{+}} \nonumber \]
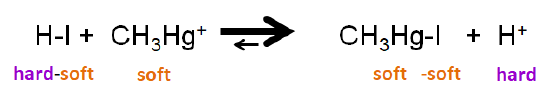 \[ \nonumber \]
\[ \nonumber \]
The hard-hard and soft-soft preferences in Lewis acid-base interactions reflect that:
- The lone pair of a hard base is strongly stabilized electrostatically by a hard acid.
- The lone pair of a soft base is strongly stabilized by forming a covalent bond with a soft acid.
- The lone pair of a hard or soft base is comparatively weakly stabilized by an acid opposite to it in hardness or softness since the overall electrostatic and covalent stabilization of the adduct is comparatively weak.
Problems
Rank the acids or bases in each set in increasing order of expected hardness.
- Cr2+ and Cr3+
- H+, Cs+, and Tl+
- SCN- (acting as a base at N) and SCN- (acting as a base at S)
- AlF3, AlH3, AlMe3
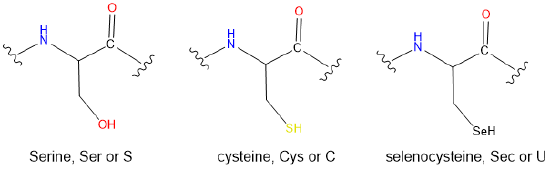
- The side chains of the following proteinogenic amino acids
- Answer
-
(a) Cr2+ < Cr3+ All other things being equal, hardness increases with oxidation state.
(b) Tl+ < Cs+ < H+ The order reflects Cs+ and Tl+'s larger size relative to H+ (which doesn't possess any electrons that can be polarized anyway) and that Tl+ still possesses two valence electrons while Cs+ possesses none.
(c) SCN- (acting as a base at S) < SCN- (acting as a base at N). The order reflects the greater electronegativity of N than S and N's possession of a more negative formal charge of -1.
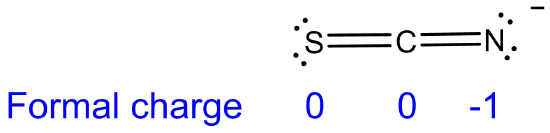
(d) AlH3 < AlMe3 < AlF3. The hardness increases as the substituents on the Lewis acid Al center become less electron donating and more electron withdrawing (and, incidentally, harder bases) as their electronegativity increases in the order H- < CH3- < F-. Note that the order of electron donating ability for H- and CH3- is the opposite observed for carbocations, for which hyperconjugation plays a larger role.
(e) Sec < Cys < Ser. The hardness increases as the electronegativity of the Lewis base chalcogen increases on going from a selenol to a thiol to an alcohol.
Predict the position of equilibrium for the following reaction.
\[\ce{Fe2O3 + 3Ag2S <=> Fe2S3 + 3Ag2O} \nonumber \]
- Answer
-
The equilibrium will favor the reactants (K<1) since the hard-hard and soft-soft interactions in the reactants are more stable than the hard-soft interactions in the products.

Predict whether \(K\) for the following equilibria will be <<1, ~1, or >>1.
- \(\ce{2HF + (CH_3Hg)_2S ⇌ 2CH_3HgF + H_2S}\)
- \(\ce{Ag(NH_3)_2^+ + 2PH_3 ⇌ Ag(PH_3)_2^+ + 2NH_3}\)
- \(\ce{Ag(PH_3)_2^+ + 2H_3B-SH_2 ⇌ 2H_3B-PH_3 + Ag(SH_2)_2^{+}}\)
- \(\ce{H_3B-NH_3 + F_3B-SH_2 ⇌ H_3B-SH_2 + F_3B-NH_3}\)
- Answer
-
a. K< < 1 since the reactant adducts are hard-hard and soft-soft while the products involve hard-soft interactions.
b. K>>1 since the reactant complex, diamine silver(I), is a complex of a hard base, NH3, with the soft acid, Ag+, while the product is a complex of the same soft acid with the soft base phosphine.
c. K~1 since all the adducts amongst the reactants and products involve soft acids and bases.
d. K>>1 since BH3 is a softer acid than BF3, so it will form a stronger complex with the softer base H2S while the harder BF3 forms a stronger complex with the harder base NH3.
Which acid will form the most stable complex with \(\ce{CO}\): \(\ce{BH3}\), \(\ce{BF3}\), or \(\ce{BMe3}\)?
- Answer
-
\(\ce{BH3}\). Since \(\ce{CO}\) forms complexes primarily through its carbon lone pair, it is a soft base and so will form the strongest complex with the softest Lewis acid.
References
1. Ahrland, S.; Chatt, J.; Davies, N. R., The relative affinities of ligand atoms for acceptor molecules and ions. Quarterly Reviews, Chemical Society 1958, 12 (3), 265-276.
2. Pearson, R. G., Hard and Soft Acids and Bases. Journal of the American Chemical Society 1963, 85 (22), 3533-3539.
3. Fleming, I., Molecular orbitals and organic chemical reactions. Reference ed.; Wiley: Hoboken, N.J., 2010.
Notes
* Despite the fruitfulness of this observation, in general it is important to reduce the potential for observer bias by checking observations like these against compounds reported in the chemical literature and databases like the Inorganic Crystal Structure and Cambridge Crystallographic Databases.
** These are very soluble in water, to the point where some solutions are perhaps better described as solutions of water in the halide.

