8.5: Phase Changes and Energy Calculations
- Page ID
- 393914
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the energy changes associated with phase changes.
- Determine the heat associated with a phase change.
- Use heating and cooling curves to show energy changes.
Matter can exist in one of several different states, including a gas, liquid, or solid state. The amount of energy in molecules of matter determines the state of matter.
- A gas is a state of matter in which atoms or molecules have enough energy to move freely. The molecules come into contact with one another only when they randomly collide.
- A liquid is a state of matter in which atoms or molecules are constantly in contact but have enough energy to keep changing positions relative to one another.
- A solid is a state of matter in which atoms or molecules do not have enough energy to move. They are constantly in contact and in fixed positions relative to one another.
Phase Changes
The following are the changes of state:
| Solid → Liquid | Melting or fusion |
| Liquid → Gas | Vaporization |
| Liquid → Solid | Freezing |
| Gas → Liquid | Condensation |
| Solid → Gas | Sublimation |
Energy Changes That Accompany Phase Changes
Phase changes are always accompanied by a change in the energy of a system. For example, converting a liquid, in which the molecules are close together, to a gas, in which the molecules are, on average, far apart, requires an input of energy (heat) to give the molecules enough kinetic energy to allow them to overcome the intermolecular attractive forces. The stronger the attractive forces, the more energy is needed to overcome them. Solids, which are highly ordered, have the strongest intermolecular interactions, whereas gases, which are very disordered, have the weakest. Thus any transition from a more ordered to a less ordered state (solid to liquid, liquid to gas, or solid to gas) requires an input of energy; it is endothermic. Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic. The energy change associated with each common phase change is shown in Figure 8.6.18.6.1.
ΔH is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state.
Previously, we defined the enthalpy changes associated with various chemical and physical processes. The melting points and molar enthalpies of fusion (ΔHfusΔHfus), the energy required to convert from a solid to a liquid, a process known as fusion (or melting), as well as the normal boiling points and enthalpies of vaporization (ΔHvapΔHvap) of selected compounds are listed in Table 8.6.18.6.1.
| Substance | Melting Point (°C) | ΔHfus (kJ/mol) | Boiling Point (°C) | ΔHvap (kJ/mol) |
|---|---|---|---|---|
| N2 | −210.0 | 0.71 | −195.8 | 5.6 |
| HCl | −114.2 | 2.00 | −85.1 | 16.2 |
| Br2 | −7.2 | 10.6 | 58.8 | 30.0 |
| CCl4 | −22.6 | 2.56 | 76.8 | 29.8 |
| CH3CH2OH (ethanol) | −114.1 | 4.93 | 78.3 | 38.6 |
| CH3(CH2)4CH3 (n-hexane) | −95.4 | 13.1 | 68.7 | 28.9 |
| H2O | 0 | 6.01 | 100 | 40.7 |
| Na | 97.8 | 2.6 | 883 | 97.4 |
| NaF | 996 | 33.4 | 1704 | 176.1 |
The substances with the highest melting points usually have the highest enthalpies of fusion; they tend to be ionic compounds that are held together by very strong electrostatic interactions. Substances with high boiling points are those with strong intermolecular interactions that must be overcome to convert a liquid to a gas, resulting in high enthalpies of vaporization. The enthalpy of vaporization of a given substance is much greater than its enthalpy of fusion because it takes more energy to completely separate molecules (conversion from a liquid to a gas) than to enable them only to move past one another freely (conversion from a solid to a liquid).
Less energy is needed to allow molecules to move past each other than to separate them totally.
Label each of the following processes as endothermic or exothermic.
- water boiling
- ice forming on a pond
Solution
- endothermic - you must put a pan of water on the stove and give it heat in order to get water to boil. Because you are adding heat/energy, the reaction is endothermic.
- exothermic - think of ice forming in your freezer instead. You put water into the freezer, which takes heat out of the water, to get it to freeze. Because heat is being pulled out of the water, it is exothermic. Heat is leaving.
Exercise \(\PageIndex{1}\)
Label each of the following processes as endothermic or exothermic.
- water vapor condensing
- gold melting
- Answer
-
a. exothermic
b. endothermic
Temperature Curves
The energy changes that occur during phase changes can be quantified by using a heating or cooling curve.
Heating Curves
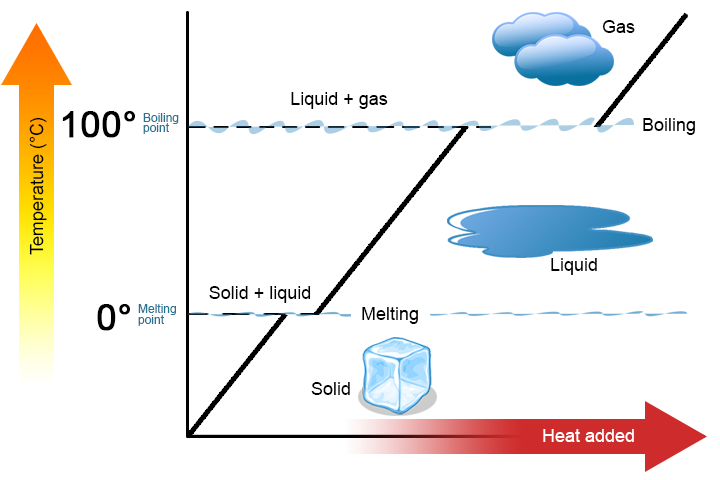
A phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a liquid to a solid). The boiling point is the temperature at which a substance goes from a liquid to a gas (or from a gas to a liquid). The nature of the phase change depends on the direction of the heat transfer. Heat going into a substance changes it from a solid to a liquid or a liquid to a gas. Removing heat from a substance changes a gas to a liquid or a liquid to a solid.
Two key points are worth emphasizing. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (H2O) as an example. On the Celsius scale, H2O has a melting point of 0°C and a boiling point of 100°C. At 0°C, both the solid and liquid phases of H2O can coexist. However, if heat is added, some of the solid H2O will melt and turn into liquid H2O. If heat is removed, the opposite happens: some of the liquid H2O turns into solid H2O. A similar process can occur at 100°C: adding heat increases the amount of gaseous H2O, while removing heat increases the amount of liquid H2O (Figure \(\PageIndex{1}\)).
Water is a good substance to use as an example because many people are already familiar with it. Other substances have melting points and boiling points as well.
Second, as shown in Figure \(\PageIndex{2}\), the temperature of a substance does not change as the substance goes from one phase to another. In other words, phase changes are isothermal (isothermal means “constant temperature”). Again, consider H2O as an example. Solid water (ice) can exist at 0°C. If heat is added to ice at 0°C, some of the solid changes phase to make liquid, which is also at 0°C. Remember, the solid and liquid phases of H2O can coexist at 0°C. Only after all of the solid has melted into liquid does the addition of heat change the temperature of the substance.
For each phase change of a substance, there is a characteristic quantity of heat needed to perform the phase change per gram (or per mole) of material. The heat of fusion (ΔHfus) is the amount of heat per gram (or per mole) required for a phase change that occurs at the melting point. The heat of vaporization (ΔHvap) is the amount of heat per gram (or per mole) required for a phase change that occurs at the boiling point. If you know the total number of grams or moles of material, you can use the ΔHfus or the ΔHvap to determine the total heat being transferred for melting or solidification using these expressions:
\[\text{heat} = n \times ΔH_{fus} \label{Eq1a} \]
where \(n\) is the number of moles and \(ΔH_{fus}\) is expressed in energy/mole or
\[\text{heat} = m \times ΔH_{fus} \label{Eq1b} \]
where \(m\) is the mass in grams and \(ΔH_{fus}\) is expressed in energy/gram.
For the boiling or condensation, use these expressions:
\[\text{heat} = n \times ΔH_{vap} \label{Eq2a} \]
where \(n\) is the number of moles) and \(ΔH_{vap}\) is expressed in energy/mole or
\[\text{heat} = m \times ΔH_{vap} \label{Eq2b} \]
where \(m\) is the mass in grams and \(ΔH_{vap}\) is expressed in energy/gram.
Remember that a phase change depends on the direction of the heat transfer. If heat transfers in, solids become liquids, and liquids become solids at the melting and boiling points, respectively. If heat transfers out, liquids solidify, and gases condense into liquids. At these points, there are no changes in temperature as reflected in the above equations.
How much heat is necessary to melt 55.8 g of ice (solid H2O) at 0°C? The heat of fusion of H2O is 79.9 cal/g.
Solution
We can use the relationship between heat and the heat of fusion (Equation \(\PageIndex{1}\)) to determine how many cal of heat are needed to melt this ice:
\[ \begin{align*} \ce{heat} &= \ce{m \times ΔH_{fus}} \\[4pt] \mathrm{heat} &= \mathrm{(55.8\: \cancel{g})\left(\dfrac{79.9\: cal}{\cancel{g}}\right)=4,460\: cal} \end{align*} \nonumber \]
How much heat is necessary to vaporize 685 g of H2O at 100°C? The heat of vaporization of H2O is 540 cal/g.
- Answer
-
\[ \begin{align*} \ce{heat} &= \ce{m \times ΔH_{vap}} \\[4pt] \mathrm{heat} &= \mathrm{(685\: \cancel{g})\left(\dfrac{540\: cal}{\cancel{g}}\right)=370,000\: cal} \end{align*} \nonumber \]
Cooling Curves
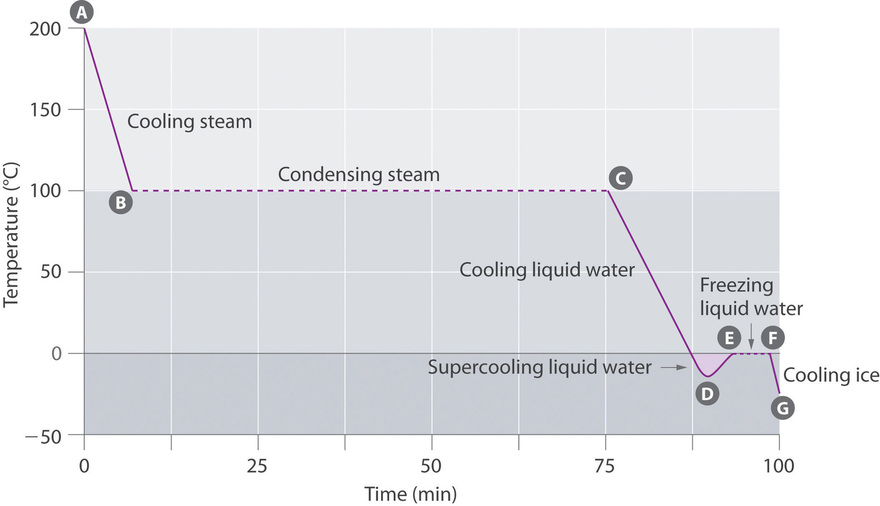
The cooling curve, a plot of temperature versus cooling time, in Figure \(\PageIndex{3}\) plots temperature versus time as a 75 g sample of steam, initially at 1 atm and 200°C, is cooled. Although we might expect the cooling curve to be the mirror image of the heating curve in Figure \(\PageIndex{2}\), the cooling curve is not an identical mirror image. As heat is removed from the steam, the temperature falls until it reaches 100°C. At this temperature, the steam begins to condense to liquid water. No further temperature change occurs until all the steam is converted to the liquid; then the temperature again decreases as the water is cooled. We might expect to reach another plateau at 0°C, where the water is converted to ice; in reality, however, this does not always occur. Instead, the temperature often drops below the freezing point for some time, as shown by the little dip in the cooling curve below 0°C. This region corresponds to an unstable form of the liquid, a supercooled liquid. If the liquid is allowed to stand, if cooling is continued, or if a small crystal of the solid phase is added (a seed crystal), the supercooled liquid will convert to a solid, sometimes quite suddenly. As the water freezes, the temperature increases slightly due to the heat evolved during the freezing process and then holds constant at the melting point as the rest of the water freezes. Subsequently, the temperature of the ice decreases again as more heat is removed from the system.
Key Takeaway
- There is an energy change associated with any phase change.


