11.5: Ionic Bonding
- Page ID
- 476583
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Predict the formulas of the ionic compounds that form according to the Octet Rule.
- Describe the structure of ionic compounds.
Does the sea really have salt in it?
We can get common table salt from several sources. It can be mined in the solid form in salt mines, or found as a solid in deposits. We can also get salt from the ocean, but it really does not exist as a salt when in solution. The sodium ions and chloride ions are dissolved, but not combined into a structure until all the water is removed.
Most of the rocks and minerals that make up the Earth's crust are composed of positive and negative ions held together by ionic bonding. An ionic compound is an electrically neutral compound consisting of positive and negative ions. You are very familiar with some ionic compounds, such as sodium chloride \(\left( \ce{NaCl} \right)\). A sodium chloride crystal consists of equal numbers of positive sodium ions \(\left( \ce{Na^+} \right)\) and negative chloride ions \(\left( \ce{Cl^-} \right)\).
Ionic Bonds
Oppositely charged particles attract each other. This attractive force is often referred to as an electrostatic force. An ionic bond is the electrostatic force that holds ions together in an ionic compound. The strength of the ionic bond is directly dependent upon the quantity of the charges and inversely dependent on the distance between the charged particles. A cation with a \(2+\) charge will make a stronger ionic bond than a cation with a \(1+\) charge. A larger ion makes a weaker ionic bond because of the greater distance between its electrons and the nucleus of the oppositely charged ion.
Electron Dot Diagrams
We will use sodium chloride as an example to demonstrate the nature of the ionic bond and how it forms. As you know, sodium is a metal and loses its one valence electron to become a cation. Chlorine is a nonmetal and gains one electron in becoming an anion. Both achieve a noble-gas electron configuration. However, electrons cannot be simply "lost" to nowhere in particular. A more accurate way to describe what is happening is that a single electron is transferred from the sodium atom to the chlorine atom, as shown below.
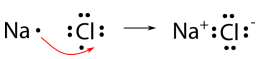
The ionic bond is the attraction of the \(\ce{Na^+}\) ion for the \(\ce{Cl^-}\) ion. It is conventional to show the cation without dots around the symbol to emphasize that the original energy level that contained the valence electron is now empty. The anion is now shown with a complete octet of electrons.
For a compound such as magnesium chloride, it is not quite as simple. Because magnesium has two valence electrons, it needs to lose both to achieve the noble-gas configuration. Therefore, two chlorine atoms will be needed.
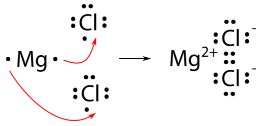
The final formula for magnesium chloride is \(\ce{MgCl_2}\).
Ionic Crystal Structure
Electron dot diagrams show the nature of the electron transfer that takes place between metal and nonmetal atoms. However, ionic compounds do not exist as discrete molecules, as the dot diagrams may suggest. In order to minimize the potential energy of the system, ionic compounds take on the form of an extended three-dimensional array of alternating cations and anions. This maximizes the attractive forces between the oppositely charged ions. The figure below shows two different ways of representing the ionic crystal lattice. A ball and stick model makes it easier to see how individual ions are oriented with respect to one another. A space filling diagram is a more accurate representation of how the ions pack together in the crystal.
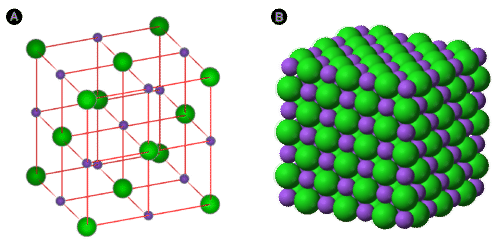
Figure \(\PageIndex{2}\): Two models of a sodium chloride crystal are shown. The purple spheres represent the \(\ce{Na^+}\) ions, while the green spheres represent the \(\ce{Cl^-}\) ions. (A) In an expanded view, the distances between ions are exaggerated, more easily showing the coordination numbers of each ion. (B) In a space filling model, the electron clouds of the ions are in contact with each other. (Credit: (A) Eloy; (B) Ben Mills (Wikimedia: Benjah-bmm27); Source: (A) wikimedia; (B) wikimedia; License: Public Domain)
Naturally occurring sodium chloride (halite) does not look at first glance like the neat diagrams shown above. It is only when we use modern techniques to analyze the crystal structure at the atomic level that we can see the true regularity of the organized ions.

Figure \(\PageIndex{3}\): Halite crystals. (Credit: Ingo Wölbern (Wikimedia: Iwoelbern); Source: wikimedia [commons.wikimedia.org]; License: Public Domain)
Section Summary
- An ionic compound contains positive and negative ions.
- An ionic bond is electrostatic in nature.
- Electron dot diagrams can be used to illustrate electron movements and ion formation.
- Ionic compounds take on the form of extended three-dimensional arrays of cations and anions.
- The arrangement maximizes the attractive force between oppositely-charged ions.
Glossary
- ionic bond
- the electrostatic force that holds ions together in an ionic compound.
- ionic compound
- a compound formed by the attraction between ions.
- crystal
- a structure of matter that is an extended three-dimensional array of repeating patterns of particles that make up that form of matter.


