11.4: Ions and Ion Formation
- Page ID
- 476580
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Use the Octet Rule to predict the charge that ions of a given element will form.
- Correctly refer to ions as anions or cations depending on their charge.
Atoms to Ions
Atoms can gain electrons. They can also lose electrons. In either case, they become ions. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. If atoms lose electrons, they become positive ions, or cations. If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine (see Figure \(\PageIndex{1}\)). A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1.
Names and Symbols
Like fluoride, other negative ions usually have names ending in –ide. Positive ions, on the other hand, are just given the element name followed by the word ion. For example, when a sodium atom loses an electron, it becomes a positive sodium ion. The charge of an ion is indicated by a plus (+) or minus sign (-), which is written to the right of and just above the ion’s chemical symbol. For example, the fluoride ion is represented by the symbol F-, and the sodium ion is represented by the symbol Na+. If the charge is greater than one, a number is used to indicate it. For example, magnesium (Mg) may lose two electrons to form an ion with a charge of plus two. This ion would be represented by the symbol Mg2+. This and some other common ions are listed with their symbols in the table below.
| Cations | Anions | ||
|---|---|---|---|
| Name of Ion | Chemical Symbol | Name of Ion | Chemical Symbol |
| Calcium ion | Ca2+ | Chloride | Cl- |
| Hydrogen ion | H+ | Fluoride | F- |
| Magnesium ion | Mg2+ | Bromide | Br- |
| Aluminum ion | Al3+ | Oxide | O2-
The process in which an atom becomes an ion is called ionization. It may occur when atoms are exposed to high levels of radiation. The radiation may give their outer electrons enough energy to escape from the attraction of the positive nucleus. However, most ions form when atoms transfer electrons to or from other atoms or |
How Ions Form molecules.
For example, sodium atoms may transfer electrons to chlorine atoms. This forms positive sodium ions (Na+) and negative chloride ions (Cl-).
Why do you think atoms lose electrons to, or gain electrons from, other atoms?
- Answer
-
Atoms form ions by losing or gaining electrons because it makes them more stable and this state takes less energy to maintain. The most stable state for an atom is to have its outermost energy level filled with the maximum possible number of electrons. In the case of metals such as lithium, with just one electron in the outermost energy level, a more stable state can be achieved by losing that one outer electron. In the case of nonmetals such as fluorine, which has seven electrons in the outermost energy level, a more stable state can be achieved by gaining one electron and filling up the outer energy level. These are two applications of the Octet Rule which we introduced earlier in this chapter.
Based on the Octet Rule, we can make some predictions for the charges that will form for elements based on their position on the periodic table. These are listed in Figure \(\PageIndex{2}\). You might notice some variability towards the bottom of the periodic table for some of these elements. These are situations where the Octet Rule is not followed and we will ignore them for this class. Look at the third or fourth period and make sure you feel comfortable predicting the charge of these elements based on their dot structure. Elements following the Octet Rule will gain or lose electrons to become isoelectronic with a noble gas. This means that they have the same number of electrons.
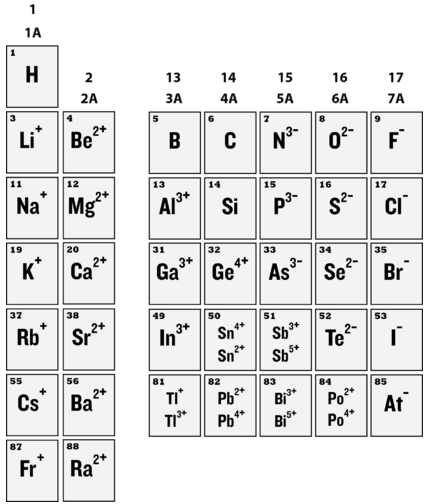
Properties of Ions
Ions are highly reactive, especially as gases. They usually react with ions of opposite charge to form neutral compounds. For example, positive sodium ions and negative chloride ions react to form the neutral compound sodium chloride, commonly known as table salt. This occurs because oppositely charged ions attract each other. Ions with the same charge, on the other hand, repel each other. Ions are also deflected by a magnetic field, as you saw in the opening image of the northern lights.
Section Summary
- Atoms have equal numbers of positive protons and negative electrons, so they are neutral in electric charge.
- Atoms can gain or lose electrons and become ions, which are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons.
- Anions are negative ions formed by accepting electrons.
- Cations form when an atom loses one or more electrons.
- The outermost principal energy level of an anion is usually an octet.
- The process in which an atom becomes an ion is called ionization. It may occur when atoms are exposed to high levels of radiation or when atoms transfer electrons to or from other atoms.
- Ions are reactive, attracted or repulsed by other charged particles, and deflected by a magnetic field.
Glossary
- anions
- negative ions that form when atoms gain electrons.
- cations
- positive ions that form when atoms lose electrons.
- ions
- atoms that have a positive or negative charge because they have unequal numbers of protons and electrons.


