9.E: Gases (Exercises)
- Last updated
- Save as PDF
- Page ID
- 112860
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)9.1: Gas Pressure
Q9.1.1
Why are sharp knives more effective than dull knives (Hint: think about the definition of pressure)?
S9.1.1
The cutting edge of a knife that has been sharpened has a smaller surface area than a dull knife. Since pressure is force per unit area, a sharp knife will exert a higher pressure with the same amount of force and cut through material more effectively.
Q9.1.2
Why do some small bridges have weight limits that depend on how many wheels or axles the crossing vehicle has?
Q9.1.3
Why should you roll or belly-crawl rather than walk across a thinly-frozen pond?
S9.1.3
Lying down distributes your weight over a larger surface area, exerting less pressure on the ice compared to standing up. If you exert less pressure, you are less likely to break through thin ice.
Q9.1.4
A typical barometric pressure in Redding, California, is about 750 mm Hg. Calculate this pressure in atm and kPa.
Q9.1.5
A typical barometric pressure in Denver, Colorado, is 615 mm Hg. What is this pressure in atmospheres and kilopascals?
S9.1.5
0.809 atm; 82.0 kPa
Q9.1.6
A typical barometric pressure in Kansas City is 740 torr. What is this pressure in atmospheres, in millimeters of mercury, and in kilopascals?
Q9.1.7
Canadian tire pressure gauges are marked in units of kilopascals. What reading on such a gauge corresponds to 32 psi?
S9.1.7
2.2 × 102 kPa
Q9.1.8
During the Viking landings on Mars, the atmospheric pressure was determined to be on the average about 6.50 millibars (1 bar = 0.987 atm). What is that pressure in torr and kPa?
Q9.1.9
The pressure of the atmosphere on the surface of the planet Venus is about 88.8 atm. Compare that pressure in psi to the normal pressure on earth at sea level in psi.
S9.1.9
Earth: 14.7 lb in–2; Venus: 13.1× 103 lb in−2
Q9.1.10
A medical laboratory catalog describes the pressure in a cylinder of a gas as 14.82 MPa. What is the pressure of this gas in atmospheres and torr?
Q9.1.11
Consider this scenario and answer the following questions: On a mid-August day in the northeastern United States, the following information appeared in the local newspaper: atmospheric pressure at sea level 29.97 in., 1013.9 mbar.
- What was the pressure in kPa?
- The pressure near the seacoast in the northeastern United States is usually reported near 30.0 in. Hg. During a hurricane, the pressure may fall to near 28.0 in. Hg. Calculate the drop in pressure in torr.
S9.1.11
(a) 101.5 kPa; (b) 51 torr drop
Q9.1.12
Why is it necessary to use a nonvolatile liquid in a barometer or manometer?
Q9.1.13
The pressure of a sample of gas is measured at sea level with a closed-end manometer. The liquid in the manometer is mercury. Determine the pressure of the gas in:
- torr
- Pa
- bar
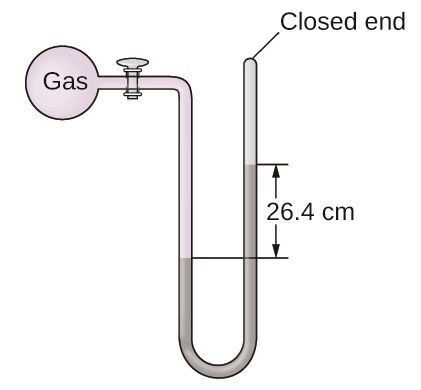
S9.1.13
(a) 264 torr; (b) 35,200 Pa; (c) 0.352 bar
Q9.1.14
The pressure of a sample of gas is measured with an open-end manometer, partially shown to the right. The liquid in the manometer is mercury. Assuming atmospheric pressure is 29.92 in. Hg, determine the pressure of the gas in:
- torr
- Pa
- bar
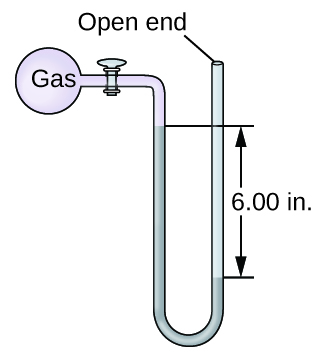
Q9.1.15
The pressure of a sample of gas is measured at sea level with an open-end mercury manometer. Assuming atmospheric pressure is 760.0 mm Hg, determine the pressure of the gas in:
- mm Hg
- atm
- kPa
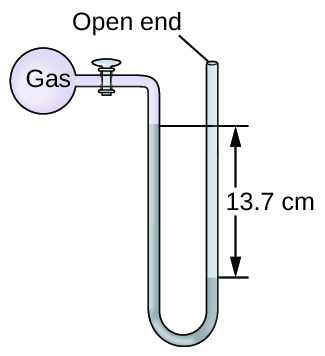
S9.1.15
(a) 623 mm Hg; (b) 0.820 atm; (c) 83.1 kPa
Q9.1.16
The pressure of a sample of gas is measured at sea level with an open-end mercury manometer. Assuming atmospheric pressure is 760 mm Hg, determine the pressure of the gas in:
- mm Hg
- atm
- kPa
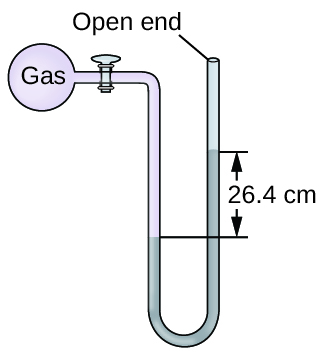
Q9.1.17
How would the use of a volatile liquid affect the measurement of a gas using open-ended manometers vs. closed-end manometers?
S9.1.17
With a closed-end manometer, no change would be observed, since the vaporized liquid would contribute equal, opposing pressures in both arms of the manometer tube. However, with an open-ended manometer, a higher pressure reading of the gas would be obtained than expected, since Pgas = Patm + Pvol liquid.
9.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
Q9.2.1
Sometimes leaving a bicycle in the sun on a hot day will cause a blowout. Why?
Q9.2.2
Explain how the volume of the bubbles exhausted by a scuba diver (Figure) change as they rise to the surface, assuming that they remain intact.
S9.2.2
As the bubbles rise, the pressure decreases, so their volume increases as suggested by Boyle’s law.
Q9.2.3
One way to state Boyle’s law is “All other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) What is the meaning of the term “inversely proportional?” (b) What are the “other things” that must be equal?
Q9.2.4
An alternate way to state Avogadro’s law is “All other things being equal, the number of molecules in a gas is directly proportional to the volume of the gas.”
- What is the meaning of the term “directly proportional?”
- What are the “other things” that must be equal?
S9.2.4
(a) The number of particles in the gas increases as the volume increases. (b) temperature, pressure
Q9.2.5
How would the graph in Figure change if the number of moles of gas in the sample used to determine the curve were doubled?
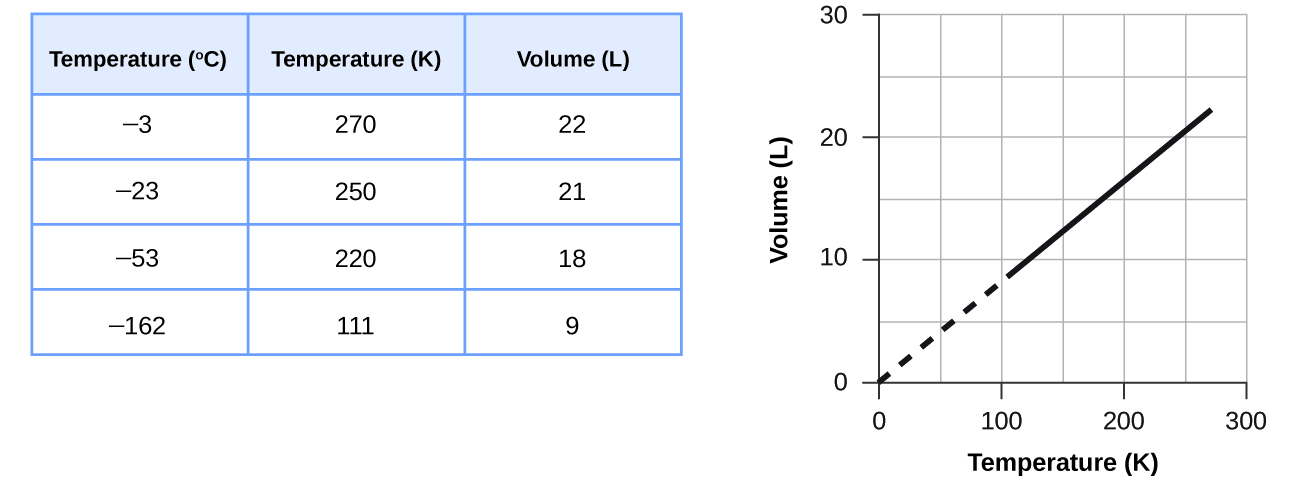
The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm. If the temperature is in kelvin, volume and temperature are directly proportional. The line stops at 111 K because methane liquefies at this temperature; when extrapolated, it intersects the graph’s origin, representing a temperature of absolute zero.
Q9.2.6
How would the graph in Figure change if the number of moles of gas in the sample used to determine the curve were doubled?
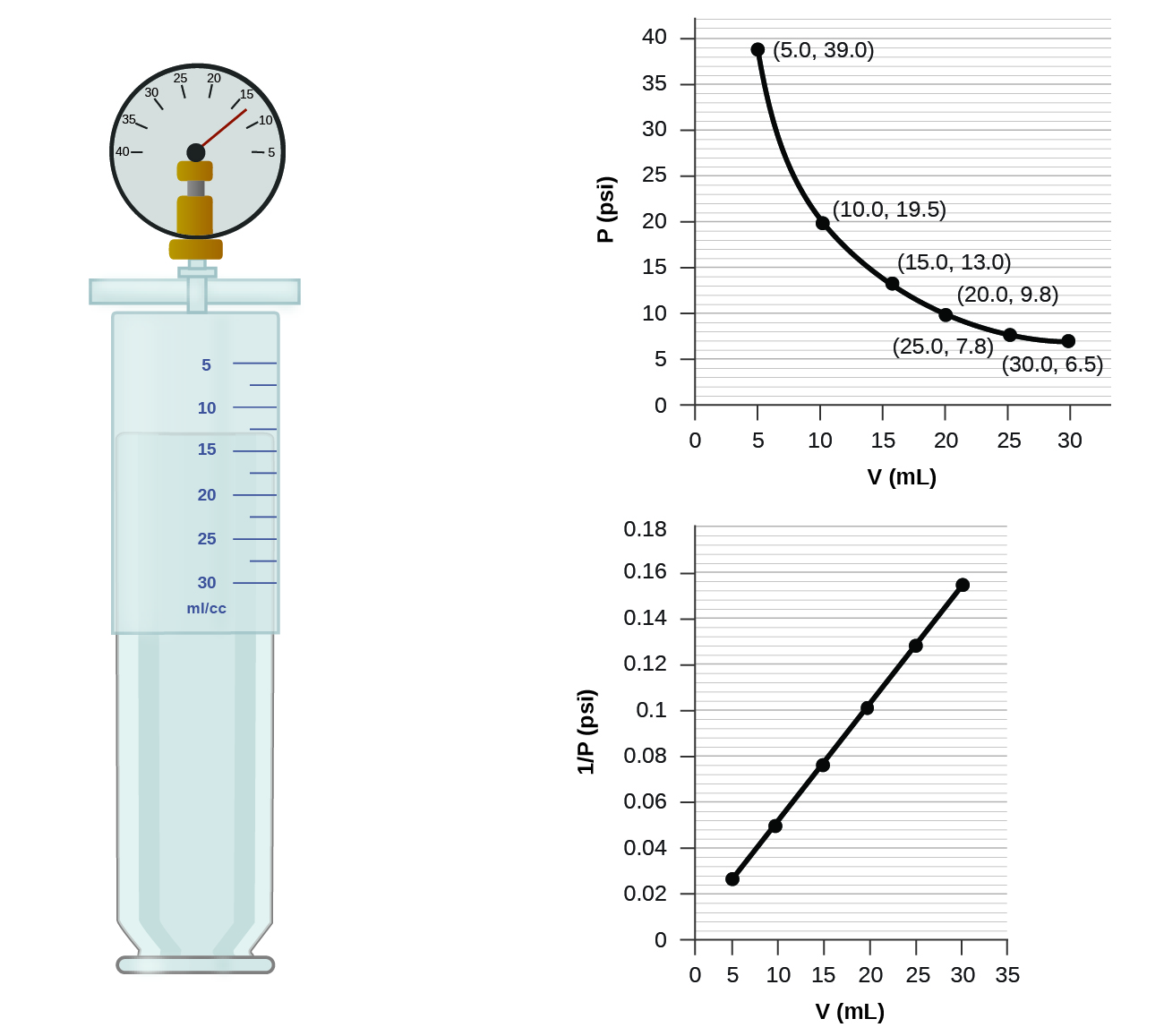
When a gas occupies a smaller volume, it exerts a higher pressure; when it occupies a larger volume, it exerts a lower pressure (assuming the amount of gas and the temperature do not change). Since P and V are inversely proportional, a graph of \(1/P\) vs. \(V\) is linear.
S9.2.6
The curve would be farther to the right and higher up, but the same basic shape.
Q9.2.7
In addition to the data found in Figure, what other information do we need to find the mass of the sample of air used to determine the graph?
Q9.2.8
Determine the volume of 1 mol of CH4 gas at 150 K and 1 atm, using Figure.
S9.2.8
16.3 to 16.5 L
Q9.2.9
Determine the pressure of the gas in the syringe shown in Figure when its volume is 12.5 mL, using:
- the appropriate graph
- Boyle’s law
Q9.2.10
A spray can is used until it is empty except for the propellant gas, which has a pressure of 1344 torr at 23 °C. If the can is thrown into a fire (T = 475 °C), what will be the pressure in the hot can?
S9.2.10
3.40 × 103 torr
Q9.2.11
What is the temperature of an 11.2-L sample of carbon monoxide, CO, at 744 torr if it occupies 13.3 L at 55 °C and 744 torr?
S9.2.11
we must use \(\dfrac{P_1V_1}{T_1} =\dfrac{P_2V_2}{T_2}\) and solve for \(T_1\)
\(T_1 = \dfrac{P_1V_1T_2}{P_2V_2}\)
Where:
\(P_1 = 744\: torr\)
\(V_1 = 11.2\: L\)
\(P_2 = 744\: torr\)
\(V_2 = 13.3\: L\)
\(T_2 = 328.15°\: K\)
\(\dfrac{(744\: torr)(11.2\: L)(328.15°\: K)}{(744\: torr)(13.3\: L)} = 276°\: K\)
276°K ; 3°C
Q9.2.12
A 2.50-L volume of hydrogen measured at –196 °C is warmed to 100 °C. Calculate the volume of the gas at the higher temperature, assuming no change in pressure.
S9.2.13
12.1 L
Q9.2.14
A balloon inflated with three breaths of air has a volume of 1.7 L. At the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Q9.2.15
A weather balloon contains 8.80 moles of helium at a pressure of 0.992 atm and a temperature of 25 °C at ground level. What is the volume of the balloon under these conditions?

S9.2.15
217 L
Q9.2.16
The volume of an automobile air bag was 66.8 L when inflated at 25 °C with 77.8 g of nitrogen gas. What was the pressure in the bag in kPa?Q9.2.17
How many moles of gaseous boron trifluoride, BF3, are contained in a 4.3410-L bulb at 788.0 K if the pressure is 1.220 atm? How many grams of BF3?
S9.2.17
8.190 × 10–2 mol; 5.553 g
Q9.2.18
Iodine, I2, is a solid at room temperature but sublimes (converts from a solid into a gas) when warmed. What is the temperature in a 73.3-mL bulb that contains 0.292 g of I2 vapor at a pressure of 0.462 atm?
S9.2.18
1.) Use the equation \(PV =nRT\) and solve for \(T\)
\(T= \dfrac{PV}{nR}\)
2.) convert grams of I2 to moles of I2 and convert mL to L
\(0.292g\: \ce{I2}\times \dfrac{1\: mole\: \ce{I2}}{253.8g\: \ce{I2}} = 1.15 \times10^{-3}\: moles\: \ce{I2}\)
\(73.3\:mL = 0.0733\:L\)
3.) Use these values along with \(R= 0.08206\: \dfrac{atm\:L}{mole\:°K}\) to solve for \(T\)
\(T= \dfrac{(0.462\: \cancel{atm})(0.0733\:\cancel{L})}{(1.15\times10^{-3}\: \cancel{moles})(0.08206\: \dfrac{\cancel{atm}\:\cancel{L}}{\cancel{mole}\:°K})} = 359\: °K \)
359°K ; 86°C
Q9.2.19
How many grams of gas are present in each of the following cases?
- 0.100 L of CO2 at 307 torr and 26 °C
- 8.75 L of C2H4, at 378.3 kPa and 483 K
- 221 mL of Ar at 0.23 torr and –54 °C
S9.2.19
(a) 7.24 × 10–2 g; (b) 23.1 g; (c) 1.5 × 10–4 g
Q9.2.20
A high altitude balloon is filled with 1.41 × 104 L of hydrogen at a temperature of 21 °C and a pressure of 745 torr. What is the volume of the balloon at a height of 20 km, where the temperature is –48 °C and the pressure is 63.1 torr?
Q9.2.21
A cylinder of medical oxygen has a volume of 35.4 L, and contains O2 at a pressure of 151 atm and a temperature of 25 °C. What volume of O2 does this correspond to at normal body conditions, that is, 1 atm and 37 °C?
S9.2.21
5561 L
Q9.2.22
A large scuba tank (Figure) with a volume of 18 L is rated for a pressure of 220 bar. The tank is filled at 20 °C and contains enough air to supply 1860 L of air to a diver at a pressure of 2.37 atm (a depth of 45 feet). Was the tank filled to capacity at 20 °C?
Q9.2.23
A 20.0-L cylinder containing 11.34 kg of butane, C4H10, was opened to the atmosphere. Calculate the mass of the gas remaining in the cylinder if it were opened and the gas escaped until the pressure in the cylinder was equal to the atmospheric pressure, 0.983 atm, and a temperature of 27 °C.
S9.2.23
46.4 g
Q9.2.24
While resting, the average 70-kg human male consumes 14 L of pure O2 per hour at 25 °C and 100 kPa. How many moles of O2 are consumed by a 70 kg man while resting for 1.0 h?
Q9.2.25
For a given amount of gas showing ideal behavior, draw labeled graphs of:
- the variation of P with V
- the variation of V with T
- the variation of P with T
- the variation of \(\dfrac{1}{P}\) with V
Q9.2.26
For a gas exhibiting ideal behavior:
Q9.2.27
A liter of methane gas, CH4, at STP contains more atoms of hydrogen than does a liter of pure hydrogen gas, H2, at STP. Using Avogadro’s law as a starting point, explain why.
Q9.2.28
The effect of chlorofluorocarbons (such as CCl2F2) on the depletion of the ozone layer is well known. The use of substitutes, such as CH3CH2F(g), for the chlorofluorocarbons, has largely corrected the problem. Calculate the volume occupied by 10.0 g of each of these compounds at STP:
- CCl2F2(g)
- CH3CH2F(g)
S9.2.28
(a) 1.85 L CCl2F2; (b) 4.66 L CH3CH2F
Q9.2.29
As 1 g of the radioactive element radium decays over 1 year, it produces 1.16 × 1018 alpha particles (helium nuclei). Each alpha particle becomes an atom of helium gas. What is the pressure in pascal of the helium gas produced if it occupies a volume of 125 mL at a temperature of 25 °C?
Q9.2.30
A balloon that is 100.21 L at 21 °C and 0.981 atm is released and just barely clears the top of Mount Crumpet in British Columbia. If the final volume of the balloon is 144.53 L at a temperature of 5.24 °C, what is the pressure experienced by the balloon as it clears Mount Crumpet?
S9.2.30
0.644 atm
Q9.2.31
If the temperature of a fixed amount of a gas is doubled at constant volume, what happens to the pressure?
Q9.2.32
If the volume of a fixed amount of a gas is tripled at constant temperature, what happens to the pressure?
S9.2.32
The pressure decreases by a factor of 3.
9.3: Stoichiometry of Gaseous Substances, Mixtures, and Reactions
Q9.3.1
What is the density of laughing gas, dinitrogen monoxide, N2O, at a temperature of 325 K and a pressure of 113.0 kPa?
S9.3.1
1.) First convert kPa to atm
\(113.0\:kPa\times\dfrac{1\:atm}{101.325\:kPa}=1.115\:atm\)
2.) The use the equation \(d=\dfrac{PM}{RT}\) where d = density in g L-1 and M = molar mass in g mol-1
\(d=\dfrac{(1.115\:atm)(44.02\dfrac{g}{\cancel{mol}})}{(0.08206\: \dfrac{\cancel{atm}\:L}{\cancel{mole}\:\cancel{°K}})(325\:\cancel{°K})}=1.84\:\dfrac{g}{L}\)
Q9.3.2
Calculate the density of Freon 12, CF2Cl2, at 30.0 °C and 0.954 atm.
S9.3.2
4.64 g L−1
Q9.3.3
Which is denser at the same temperature and pressure, dry air or air saturated with water vapor? Explain.
Q9.3.4
A cylinder of O2(g) used in breathing by emphysema patients has a volume of 3.00 L at a pressure of 10.0 atm. If the temperature of the cylinder is 28.0 °C, what mass of oxygen is in the cylinder?
S9.3.4
38.8 g
Q9.3.5
What is the molar mass of a gas if 0.0494 g of the gas occupies a volume of 0.100 L at a temperature 26 °C and a pressure of 307 torr?
S9.3.5
1.) convert torr to atm and °C to °K
\(307\:torr=0.404atm\)
\(26°C= 300.°K\)
2.) Use the equation \(PV=nRT\) and solve for \(n\)
\(n=\dfrac{PV}{RT}\)
\(n=\dfrac{(0.404\:\cancel{atm})(0.100\:\cancel{L})}{(0.08206\dfrac{\cancel{atm}\:\cancel{L}}{mol\:\cancel{°K}})(300.\cancel{°K})}=0.00165\:moles\)
3.) Then divide grams by the number of moles to obtain the molar mass:
\(\dfrac{0.0494g}{0.00165\:moles}=30.0\dfrac{g}{mole}\)
Q9.3.6
What is the molar mass of a gas if 0.281 g of the gas occupies a volume of 125 mL at a temperature 126 °C and a pressure of 777 torr?
S9.3.6
72.0 g mol−1
Q9.3.7
How could you show experimentally that the molecular formula of propene is C3H6, not CH2?
Q9.3.8
The density of a certain gaseous fluoride of phosphorus is 3.93 g/L at STP. Calculate the molar mass of this fluoride and determine its molecular formula.
S9.3.8
88.1 g mol−1; PF3
Q9.3.9
Consider this question: What is the molecular formula of a compound that contains 39% C, 45% N, and 16% H if 0.157 g of the compound occupies 125 mL with a pressure of 99.5 kPa at 22 °C?
- Outline the steps necessary to answer the question.
- Answer the question.
Q9.3.10
A 36.0–L cylinder of a gas used for calibration of blood gas analyzers in medical laboratories contains 350 g CO2, 805 g O2, and 4,880 g N2. At 25 degrees C, what is the pressure in the cylinder in atmospheres?
S9.3.10
141 atm
Q9.3.11
A cylinder of a gas mixture used for calibration of blood gas analyzers in medical laboratories contains 5.0% CO2, 12.0% O2, and the remainder N2 at a total pressure of 146 atm. What is the partial pressure of each component of this gas? (The percentages given indicate the percent of the total pressure that is due to each component.)
Q9.3.12
A sample of gas isolated from unrefined petroleum contains 90.0% CH4, 8.9% C2H6, and 1.1% C3H8 at a total pressure of 307.2 kPa. What is the partial pressure of each component of this gas? (The percentages given indicate the percent of the total pressure that is due to each component.)
S9.3.12
CH4: 276 kPa; C2H6: 27 kPa; C3H8: 3.4 kPa
Q9.3.13
A mixture of 0.200 g of H2, 1.00 g of N2, and 0.820 g of Ar is stored in a closed container at STP. Find the volume of the container, assuming that the gases exhibit ideal behavior.
Q9.3.14
Most mixtures of hydrogen gas with oxygen gas are explosive. However, a mixture that contains less than 3.0 % O2 is not. If enough O2 is added to a cylinder of H2 at 33.2 atm to bring the total pressure to 34.5 atm, is the mixture explosive?
S9.3.14
Yes
Q9.3.15
A commercial mercury vapor analyzer can detect, in air, concentrations of gaseous Hg atoms (which are poisonous) as low as 2 × 10−6 mg/L of air. At this concentration, what is the partial pressure of gaseous mercury if the atmospheric pressure is 733 torr at 26 °C?
Q9.3.16
A sample of carbon monoxide was collected over water at a total pressure of 756 torr and a temperature of 18 °C. What is the pressure of the carbon monoxide? (See Table for the vapor pressure of water.)
S9.3.16
740 torr
Q9.3.17
In an experiment in a general chemistry laboratory, a student collected a sample of a gas over water. The volume of the gas was 265 mL at a pressure of 753 torr and a temperature of 27 °C. The mass of the gas was 0.472 g. What was the molar mass of the gas?
Q9.3.18
Joseph Priestley first prepared pure oxygen by heating mercuric oxide, HgO:
\(\ce{2HgO}(s)⟶\ce{2Hg}(l)+\ce{O2}(g)\)
- Outline the steps necessary to answer the following question: What volume of O2 at 23 °C and 0.975 atm is produced by the decomposition of 5.36 g of HgO?
- Answer the question.
S9.3.18
(a) Determine the moles of HgO that decompose; using the chemical equation, determine the moles of O2 produced by decomposition of this amount of HgO; and determine the volume of O2 from the moles of O2, temperature, and pressure. (b) 0.308 L
Q9.3.19
Cavendish prepared hydrogen in 1766 by the novel method of passing steam through a red-hot gun barrel:
\[\ce{4H2O}(g)+\ce{3Fe}(s)⟶\ce{Fe3O4}(s)+\ce{4H2}(g)\]
- Outline the steps necessary to answer the following question: What volume of H2 at a pressure of 745 torr and a temperature of 20 °C can be prepared from the reaction of 15.O g of H2O?
- Answer the question.
Q9.3.20
The chlorofluorocarbon CCl2F2 can be recycled into a different compound by reaction with hydrogen to produce CH2F2(g), a compound useful in chemical manufacturing:\[\ce{CCl2F2}(g)+\ce{4H2}(g)⟶\ce{CH2F2}(g)+\ce{2HCl}(g)\]
- Outline the steps necessary to answer the following question: What volume of hydrogen at 225 atm and 35.5 °C would be required to react with 1 ton (1.000 × 103 kg) of CCl2F2?
- Answer the question.
S9.3.20
- Determine the molar mass of CCl2F2. From the balanced equation, calculate the moles of H2 needed for the complete reaction. From the ideal gas law, convert moles of H2 into volume.
- 3.72 × 103 L
Q9.3.21
Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (NaN3). The other product is sodium metal. Calculate the volume of nitrogen gas at 27 °C and 756 torr formed by the decomposition of 125 g of sodium azide.
Q9.3.22
Lime, CaO, is produced by heating calcium carbonate, CaCO3; carbon dioxide is the other product.
- Outline the steps necessary to answer the following question: What volume of carbon dioxide at 875° and 0.966 atm is produced by the decomposition of 1 ton (1.000 × 103 kg) of calcium carbonate?
- Answer the question.
S9.3.22
(a) Balance the equation. Determine the grams of CO2 produced and the number of moles. From the ideal gas law, determine the volume of gas. (b) 7.43 × 105 L
Q9.3.23
Before small batteries were available, carbide lamps were used for bicycle lights. Acetylene gas, C2H2, and solid calcium hydroxide were formed by the reaction of calcium carbide, CaC2, with water. The ignition of the acetylene gas provided the light. Currently, the same lamps are used by some cavers, and calcium carbide is used to produce acetylene for carbide cannons.
- Outline the steps necessary to answer the following question: What volume of C2H2 at 1.005 atm and 12.2 °C is formed by the reaction of 15.48 g of CaC2 with water?
- Answer the question.
Q9.3.24
Calculate the volume of oxygen required to burn 12.00 L of ethane gas, C2H6, to produce carbon dioxide and water, if the volumes of C2H6 and O2 are measured under the same conditions of temperature and pressure.
S9.3.24
42.00 L
Q9.3.25
What volume of O2 at STP is required to oxidize 8.0 L of NO at STP to NO2? What volume of NO2 is produced at STP?
Q9.3.26
Consider the following questions:
- What is the total volume of the CO2(g) and H2O(g) at 600 °C and 0.888 atm produced by the combustion of 1.00 L of C2H6(g) measured at STP?
- What is the partial pressure of H2O in the product gases?
S9.3.26
(a) 18.0 L; (b) 0.533 atm
Q9.3.27
Methanol, CH3OH, is produced industrially by the following reaction:
\[\ce{CO}(g)+\ce{2H2}(g)\xrightarrow{\textrm{ copper catalyst 300 °C, 300 atm }}\ce{CH3OH}(g)\]
Q9.3.28
Assuming that the gases behave as ideal gases, find the ratio of the total volume of the reactants to the final volume.
Q9.3.29
What volume of oxygen at 423.0 K and a pressure of 127.4 kPa is produced by the decomposition of 129.7 g of BaO2 to BaO and O2?
S9.3.29
10.57 L O2
Q9.3.30
A 2.50-L sample of a colorless gas at STP decomposed to give 2.50 L of N2 and 1.25 L of O2 at STP. What is the colorless gas?
Q9.3.31
Ethanol, C2H5OH, is produced industrially from ethylene, C2H4, by the following sequence of reactions:
\[\ce{3C2H4 + 2H2SO4⟶C2H5HSO4 + (C2H5)2SO4}\]
\[\ce{C2H5HSO4 + (C2H5)2SO4 + 3H2O⟶3C2H5OH + 2H2SO4}\]
What volume of ethylene at STP is required to produce 1.000 metric ton (1000 kg) of ethanol if the overall yield of ethanol is 90.1%?
S9.3.31
5.40 × 105 L
Q9.3.32
One molecule of hemoglobin will combine with four molecules of oxygen. If 1.0 g of hemoglobin combines with 1.53 mL of oxygen at body temperature (37 °C) and a pressure of 743 torr, what is the molar mass of hemoglobin?
Q9.3.33
A sample of a compound of xenon and fluorine was confined in a bulb with a pressure of 18 torr. Hydrogen was added to the bulb until the pressure was 72 torr. Passage of an electric spark through the mixture produced Xe and HF. After the HF was removed by reaction with solid KOH, the final pressure of xenon and unreacted hydrogen in the bulb was 36 torr. What is the empirical formula of the xenon fluoride in the original sample? (Note: Xenon fluorides contain only one xenon atom per molecule.)
S9.3.33
XeF2
Q9.3.34
One method of analyzing amino acids is the van Slyke method. The characteristic amino groups (−NH2) in protein material are allowed to react with nitrous acid, HNO2, to form N2 gas. From the volume of the gas, the amount of amino acid can be determined. A 0.0604-g sample of a biological sample containing glycine, CH2(NH2)COOH, was analyzed by the van Slyke method and yielded 3.70 mL of N2 collected over water at a pressure of 735 torr and 29 °C. What was the percentage of glycine in the sample?
\(\ce{CH2(NH2)CO2H + HNO2⟶CH2(OH)CO2H + H2O + N2}\)9.4: Effusion and Diffusion of Gases
Q9.4.1
A balloon filled with helium gas is found to take 6 hours to deflate to 50% of its original volume. How long will it take for an identical balloon filled with the same volume of hydrogen gas (instead of helium) to decrease its volume by 50%?
S9.4.1
4.2 hours
Q9.4.2
Explain why the numbers of molecules are not identical in the left- and right-hand bulbs shown in the center illustration of Figure.
Q9.4.3
Starting with the definition of rate of effusion and Graham’s finding relating rate and molar mass, show how to derive the Graham’s law equation, relating the relative rates of effusion for two gases to their molecular masses.
S9.4.3
Effusion can be defined as the process by which a gas escapes through a pinhole into a vacuum. Graham’s law states that with a mixture of two gases A and B: \(\mathrm{\left(\dfrac{rate\: A}{rate\: B}\right)=\left(\dfrac{molar\: mass\: of\: B}{molar\: mass\: of\: A}\right)^{1/2}}\). Both A and B are in the same container at the same temperature, and therefore will have the same kinetic energy:
\(\mathrm{KE_A=KE_BKE}=\dfrac{1}{2}mv^2\) Therefore, \(\dfrac{1}{2}m_\ce{A}v^2_\ce{A}=\dfrac{1}{2}m_\ce{B}v^2_\ce{B}\) \(\dfrac{v^2_\ce{A}}{v^2_\ce{B}}=\dfrac{m_\ce{B}}{m_\ce{A}}\) \(\left(\dfrac{v^2_\ce{A}}{v^2_\ce{B}}\right)^{1/2}=\left(\dfrac{m_\ce{B}}{m_\ce{A}}\right)^{1/2}\) \(\dfrac{v_\ce{A}}{v_\ce{B}}=\left(\dfrac{m_\ce{B}}{m_\ce{A}}\right)^{1/2}\)Q9.4.4
Heavy water, D2O (molar mass = 20.03 g mol–1), can be separated from ordinary water, H2O (molar mass = 18.01), as a result of the difference in the relative rates of diffusion of the molecules in the gas phase. Calculate the relative rates of diffusion of H2O and D2O.
Q9.4.5
Which of the following gases diffuse more slowly than oxygen? F2, Ne, N2O, C2H2, NO, Cl2, H2S
S9.4.5
F2, N2O, Cl2, H2S
Q9.4.6
During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235UF6 diffuses 0.4% faster than 238UF6. Show the calculation that supports this value. The molar mass of 235UF6 = 235.043930 + 6 × 18.998403 = 349.034348 g/mol, and the molar mass of 238UF6 = 238.050788 + 6 × 18.998403 = 352.041206 g/mol.
Q9.4.7
Calculate the relative rate of diffusion of 1H2 (molar mass 2.0 g/mol) compared to that of 2H2 (molar mass 4.0 g/mol) and the relative rate of diffusion of O2 (molar mass 32 g/mol) compared to that of O3 (molar mass 48 g/mol).
S9.4.7
1.4; 1.2
Q9.4.8
A gas of unknown identity diffuses at a rate of 83.3 mL/s in a diffusion apparatus in which carbon dioxide diffuses at the rate of 102 mL/s. Calculate the molecular mass of the unknown gas.
Q9.4.9
When two cotton plugs, one moistened with ammonia and the other with hydrochloric acid, are simultaneously inserted into opposite ends of a glass tube that is 87.0 cm long, a white ring of NH4Cl forms where gaseous NH3 and gaseous HCl first come into contact. (Hint: Calculate the rates of diffusion for both NH3 and HCl, and find out how much faster NH3 diffuses than HCl.)
\[\ce{NH3}(g)+\ce{HCl}(g)⟶\ce{NH4Cl}(s)\]Q9.4.10
At approximately what distance from the ammonia moistened plug does this occur?S9.4.10
51.7 cm
9.5: The Kinetic-Molecular Theory
Q9.5.1
Using the postulates of the kinetic molecular theory, explain why a gas uniformly fills a container of any shape.
Q9.5.2
Can the speed of a given molecule in a gas double at constant temperature? Explain your answer.
S9.5.2
Yes. At any given instant, there are a range of values of molecular speeds in a sample of gas. Any single molecule can speed up or slow down as it collides with other molecules. The average velocity of all the molecules is constant at constant temperature.
Q9.5.3
Describe what happens to the average kinetic energy of ideal gas molecules when the conditions are changed as follows:
- The pressure of the gas is increased by reducing the volume at constant temperature.
- The pressure of the gas is increased by increasing the temperature at constant volume.
- The average velocity of the molecules is increased by a factor of 2.
Q9.5.4
The distribution of molecular velocities in a sample of helium is shown in Figure. If the sample is cooled, will the distribution of velocities look more like that of H2 or of H2O? Explain your answer.
S9.5.4
H2O. Cooling slows the velocities of the He atoms, causing them to behave as though they were heavier.
Q9.5.5
What is the ratio of the average kinetic energy of a SO2 molecule to that of an O2 molecule in a mixture of two gases? What is the ratio of the root mean square speeds, urms, of the two gases?
Q9.5.6
A 1-L sample of CO initially at STP is heated to 546 °C, and its volume is increased to 2 L.
- What effect do these changes have on the number of collisions of the molecules of the gas per unit area of the container wall?
- What is the effect on the average kinetic energy of the molecules?
- What is the effect on the root mean square speed of the molecules?
S9.5.6
(a) The number of collisions per unit area of the container wall is constant. (b) The average kinetic energy doubles. (c) The root mean square speed increases to \(\sqrt{2}\) times its initial value; urms is proportional to \(\mathrm{KE_{avg}}\).
Q9.5.7
The root mean square speed of H2 molecules at 25 °C is about 1.6 km/s. What is the root mean square speed of a N2 molecule at 25 °C?
Q9.5.8
Answer the following questions:
- Is the pressure of the gas in the hot air balloon shown at the opening of this chapter greater than, less than, or equal to that of the atmosphere outside the balloon?
- Is the density of the gas in the hot air balloon shown at the opening of this chapter greater than, less than, or equal to that of the atmosphere outside the balloon?
- At a pressure of 1 atm and a temperature of 20 °C, dry air has a density of 1.2256 g/L. What is the (average) molar mass of dry air?
- The average temperature of the gas in a hot air balloon is 1.30 × 102 °F. Calculate its density, assuming the molar mass equals that of dry air.
- The lifting capacity of a hot air balloon is equal to the difference in the mass of the cool air displaced by the balloon and the mass of the gas in the balloon. What is the difference in the mass of 1.00 L of the cool air in part (c) and the hot air in part (d)?
- An average balloon has a diameter of 60 feet and a volume of 1.1 × 105 ft3. What is the lifting power of such a balloon? If the weight of the balloon and its rigging is 500 pounds, what is its capacity for carrying passengers and cargo?
- A balloon carries 40.0 gallons of liquid propane (density 0.5005 g/L). What volume of CO2 and H2O gas is produced by the combustion of this propane?
- A balloon flight can last about 90 minutes. If all of the fuel is burned during this time, what is the approximate rate of heat loss (in kJ/min) from the hot air in the bag during the flight?
S9.5.1
(a) equal; (b) less than; (c) 29.48 g mol−1; (d) 1.0966 g L−1; (e) 0.129 g/L; (f) 4.01 × 105 g; net lifting capacity = 384 lb; (g) 270 L; (h) 39.1 kJ min−1
Q9.5.1
Show that the ratio of the rate of diffusion of Gas 1 to the rate of diffusion of Gas 2, \(\dfrac{R_1}{R_2}\), is the same at 0 °C and 100 °C.9.6: Non-Ideal Gas Behavior
Q9.6.1
Graphs showing the behavior of several different gases follow. Which of these gases exhibit behavior significantly different from that expected for ideal gases?
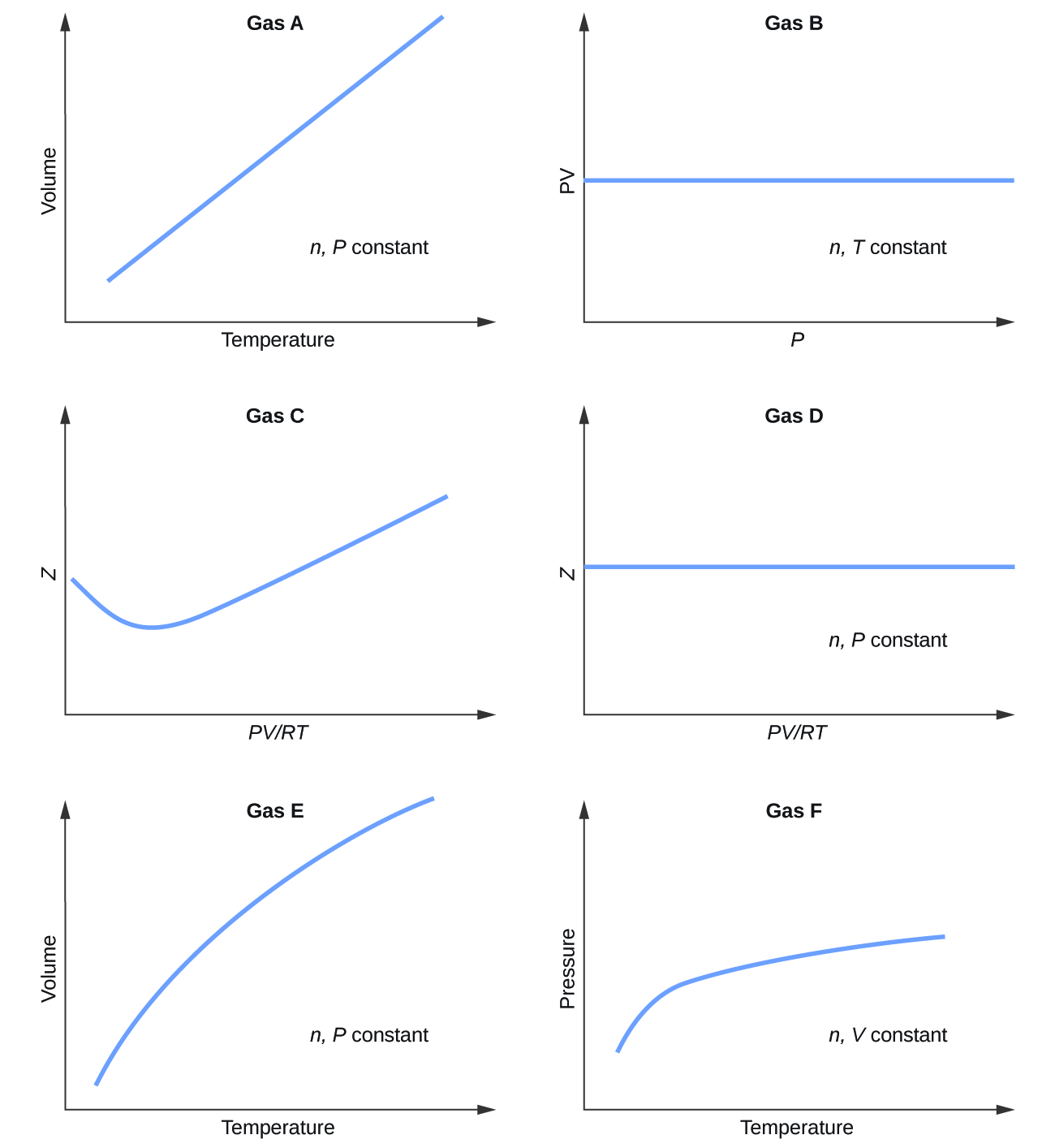
S9.6.1
Gases C, E, and F
Q9.6.3
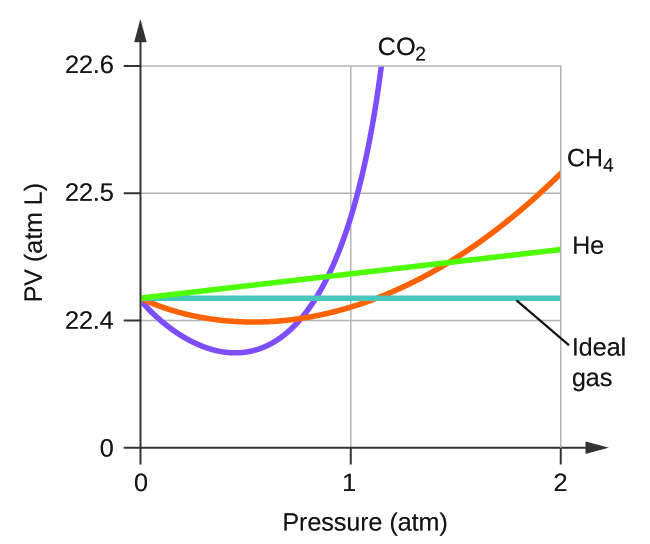
Explain why the plot of PV for CO2 differs from that of an ideal gas.
Q9.6.3
Under which of the following sets of conditions does a real gas behave most like an ideal gas, and for which conditions is a real gas expected to deviate from ideal behavior? Explain.
- high pressure, small volume
- high temperature, low pressure
- low temperature, high pressure
S9.6.3
The gas behavior most like an ideal gas will occur under the conditions in (b). Molecules have high speeds and move through greater distances between collision; they also have shorter contact times and interactions are less likely. Deviations occur with the conditions described in (a) and (c). Under conditions of (a), some gases may liquefy. Under conditions of (c), most gases will liquefy.
Q9.6.4
Describe the factors responsible for the deviation of the behavior of real gases from that of an ideal gas.
Q9.6.5
For which of the following gases should the correction for the molecular volume be largest: CO, CO2, H2, He, NH3, SF6?
S9.6.6
SF6
Q9.6.7
A 0.245-L flask contains 0.467 mol CO2 at 159 °C. Calculate the pressure:
- using the ideal gas law
- using the van der Waals equation
- Explain the reason for the difference.
- Identify which correction (that for P or V) is dominant and why.
Q9.6.8
Answer the following questions:
- If XX behaved as an ideal gas, what would its graph of Z vs. P look like?
- For most of this chapter, we performed calculations treating gases as ideal. Was this justified?
- What is the effect of the volume of gas molecules on Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
- What is the effect of intermolecular attractions on the value of Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
- In general, under what temperature conditions would you expect Z to have the largest deviations from the Z for an ideal gas?
S9.6.8
(a) A straight horizontal line at 1.0; (b) When real gases are at low pressures and high temperatures they behave close enough to ideal gases that they are approximated as such, however, in some cases, we see that at a high pressure and temperature, the ideal gas approximation breaks down and is significantly different from the pressure calculated by the van der Waals equation (c) The greater the compressibility, the more the volume matters. At low pressures, the correction factor for intermolecular attractions is more significant, and the effect of the volume of the gas molecules on Z would be a small lowering compressibility. At higher pressures, the effect of the volume of the gas molecules themselves on Z would increase compressibility (see Figure) (d) Once again, at low pressures, the effect of intermolecular attractions on Z would be more important than the correction factor for the volume of the gas molecules themselves, though perhaps still small. At higher pressures and low temperatures, the effect of intermolecular attractions would be larger. See Figure. (e) low temperatures
9.6: Non-Ideal Gas Behavior
Exercises
Graphs showing the behavior of several different gases follow. Which of these gases exhibit behavior significantly different from that expected for ideal gases?
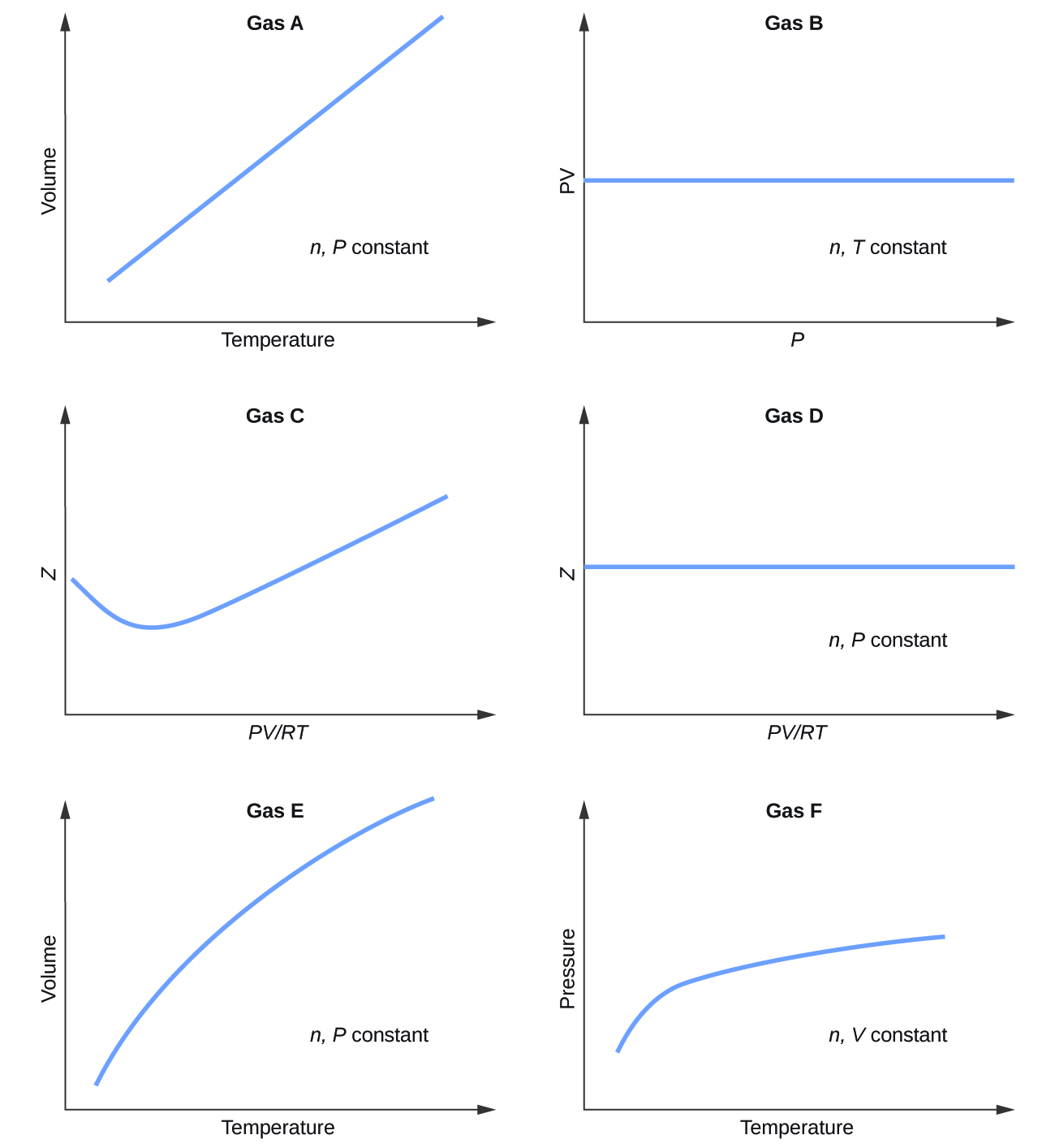
Gases C, E, and F
Explain why the plot of PV for CO2 differs from that of an ideal gas.
Under which of the following sets of conditions does a real gas behave most like an ideal gas, and for which conditions is a real gas expected to deviate from ideal behavior? Explain.
(a) high pressure, small volume
(b) high temperature, low pressure
(c) low temperature, high pressure
The gas behavior most like an ideal gas will occur under the conditions in (b). Molecules have high speeds and move through greater distances between collision; they also have shorter contact times and interactions are less likely. Deviations occur with the conditions described in (a) and (c). Under conditions of (a), some gases may liquefy. Under conditions of (c), most gases will liquefy.
Describe the factors responsible for the deviation of the behavior of real gases from that of an
ideal gas.
For which of the following gases should the correction for the molecular volume be largest:
CO, CO2, H2, He, NH3, SF6?
SF6
A 0.245-L flask contains 0.467 mol CO2 at 159 °C. Calculate the pressure:
(a) using the ideal gas law
(b) using the van der Waals equation
(c) Explain the reason for the difference.
(d) Identify which correction (that for P or V) is dominant and why.
Answer the following questions:
(a) If XX behaved as an ideal gas, what would its graph of Z vs. P look like?
(b) For most of this chapter, we performed calculations treating gases as ideal. Was this justified?
(c) What is the effect of the volume of gas molecules on Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
(d) What is the effect of intermolecular attractions on the value of Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
(e) In general, under what temperature conditions would you expect Z to have the largest deviations from the Z for an ideal gas?
(a) A straight horizontal line at 1.0; (b) When real gases are at low pressures and high temperatures they behave close enough to ideal gases that they are approximated as such, however, in some cases, we see that at a high pressure and temperature, the ideal gas approximation breaks down and is significantly different from the pressure calculated by the van der Waals equation (c) The greater the compressibility, the more the volume matters. At low pressures, the correction factor for intermolecular attractions is more significant, and the effect of the volume of the gas molecules on Z would be a small lowering compressibility. At higher pressures, the effect of the volume of the gas molecules themselves on Z would increase compressibility (see Figure) (d) Once again, at low pressures, the effect of intermolecular attractions on Z would be more important than the correction factor for the volume of the gas molecules themselves, though perhaps still small. At higher pressures and low temperatures, the effect of intermolecular attractions would be larger. See Figure. (e) low temperatures


