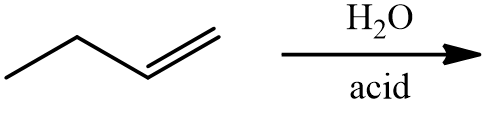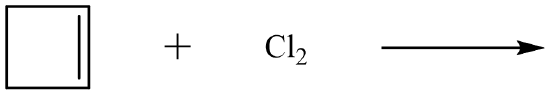5.2: Alkene Reactions
- Page ID
- 340357
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Identify and describe addition and elimination reactions.
- Distinguish between the types of addition reactions.
- Predict products of each reaction type.
- Predict products based on Markovnikov's rule.
- Define "polymer".
Organic reactions require the breaking of strong covalent bonds, which takes a considerable input of energy. In order for relatively stable organic molecules to react at a reasonable rate, they often must be modified with the use of highly reactive materials or in the presence of a catalyst. In this lesson, you will learn about several general categories of organic reactions.
Addition Reactions
Addition reactions are useful ways to introduce a new functional group into an organic molecule. An addition reaction is a reaction in which an atom or molecule is added to an unsaturated molecule, making a single product. An addition reaction can often be thought of as adding a molecule across the double bond of an alkene or across the triple bond of an alkyne.

Figure \(\PageIndex{1}\): Reaction scheme of a general alkene addition reaction.
Knowing that "ation" means to add, the specific names of these reactions, such as hydrogenation, hydration, or halogination (bromination or chlorination), should make sense. Note that hydrogenation (adding H2) and hydration (adding H2O) are very different processes.
Hydrogenation
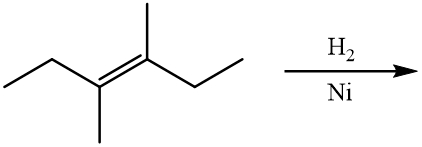
One type of addition reaction is called hydrogenation. Hydrogenation is a reaction that occurs when molecular hydrogen (H2) is added to an alkene to produce an alkane or hydrogen is added to an alkyne to produce an alkene or alkane. The reaction is typically performed in the presence of a metal catalyst such as nickel (Ni) or platinum (Pt). For example, ethene reacts with hydrogen to form ethane.
\[\ce{CH_2=CH_2} \left( g \right) + \ce{H_2} \left( g \right) \overset{\ce{Pt}}{\rightarrow} \ce{CH_3CH_3} \left( g \right)\]
Note that the hydrogenation reaction is also a redox reaction. Ethene is reduced, because the oxidation numbers of the carbon atoms change from \(-2\) to \(-3\) as a result of the reaction.
Vegetable oils consist of long carbon chains with carboxyl groups on the end; these molecules are referred to as fatty acids. The carbon chains of the fatty acids in vegetable oils are unsaturated, usually containing multiple double bonds. When hydrogen gas is blown through a sample of the oil, hydrogen atoms add across the double bonds. This conversion changes the substance from a liquid oil into a solid fat. The "hydrogenated" on a food product is an indication that oil (liquid) has been converted into fat (solid) by this process. Margarine is manufactured from unsaturated vegetable oil in this way by hydrogenating some of the double bonds making it a "partially hydrogenated vegetable oil".
Halogenation/Hydrohalogenation
Alkyl halides can be produced from an alkene by the addition of either the elemental halogen or the hydrogen halide. When the reactant is the diatomic halogen, the reaction is known as a halogenation reaction. The product of a halogenation addition reaction is a disubstituted alkyl halide as in the addition of bromine to ethene.
\[\ce{CH_2=CH_2} \left( g \right) + \ce{Br_2} \left( l \right) \rightarrow \ce{CH_2BrCH_2Br} \left( g \right)\]
The addition of bromine to an unknown organic compound can be used as a test for unsaturation in the compound. Bromine has a distinctive brownish-orange color, while most bromoalkanes are colorless. When bromine is slowly added to a solution of the compound, the orange color will fade if it undergoes an addition reaction to produce an alkyl halide. If the orange color remains, then the original compound was already saturated, and no reaction occurred.
A monosubstituted alkyl halide can be produced by the addition of a hydrogen halide to an alkene in a hydrohalogenation reaction. Shown below is the formation of chloroethane.
\[\ce{CH_2=CH_2} \left( g \right) + \ce{HCl} \left( g \right) \rightarrow \ce{CH_3CH_2Cl} \left( g \right)\]
Unlike addition reactions involving \(\ce{H_2}\), \(\ce{Br_2}\), or \(\ce{Cl_2}\), the addition of a hydrogen halide can have two possible products because an \(\ce{-H}\) and a \(\ce{-Br}\) or \(\ce{-Cl}\) are being added to the carbons in the double bond. Markovnikov's rule helps predict the major (main) product in an addition reaction involving an asymmetric alkene double bond. The rule states that the hydrogen atom from the hydrogen halide will add to the carbon that originally had more hydrogen atoms. For example, look at the reaction representing the addition of HBr to propene (Figure \(\PageIndex{2}\)).
Note that the first carbon in propene started with two hydrogen atoms and the second carbon started with one hydrogen. Therefore, the major product is formed when the hydrogen from HBr is added to the first carbon and the \(\ce{-Br}\) is added to the second carbon. When there are equal numbers of hydrogen atoms on both carbons in a double bond then the two products will form in approximately equal amounts. For example, the hydrohalogenation of 2-pentene results in two products. In the first product, the \(\ce{-Br}\) group is on the second carbon and in the second product, the \(\ce{-Br}\) group is on the third carbon. While these two molecules will have similar properties, there will be differences.

Hydration
A hydration reaction is a reaction in which water is added to an alkene. Hydration reactions can take place when the alkene and water are heated to near \(100^\text{o} \text{C}\) in the presence of a strong acid, which acts as a catalyst. Shown below is the hydration of ethene to produce ethanol.
\[\ce{CH_2=CH_2} \left( g \right) + \ce{H_2O} \left( l \right) \rightarrow \ce{CH_3CH_2OH} \left( g \right)\]
As with hydrohalogenation reactions, the addition of water can have two possible products because an \(\ce{-H}\) and an \(\ce{-OH}\) are being added to the carbons in the double bond. Therefore, Markovnikov's rule is also used to help predict the major product in a hydration reaction.
The rule states that the hydrogen atom from water will add to the carbon that originally had more hydrogen atoms. For example, look at the hydration of 1-butene.
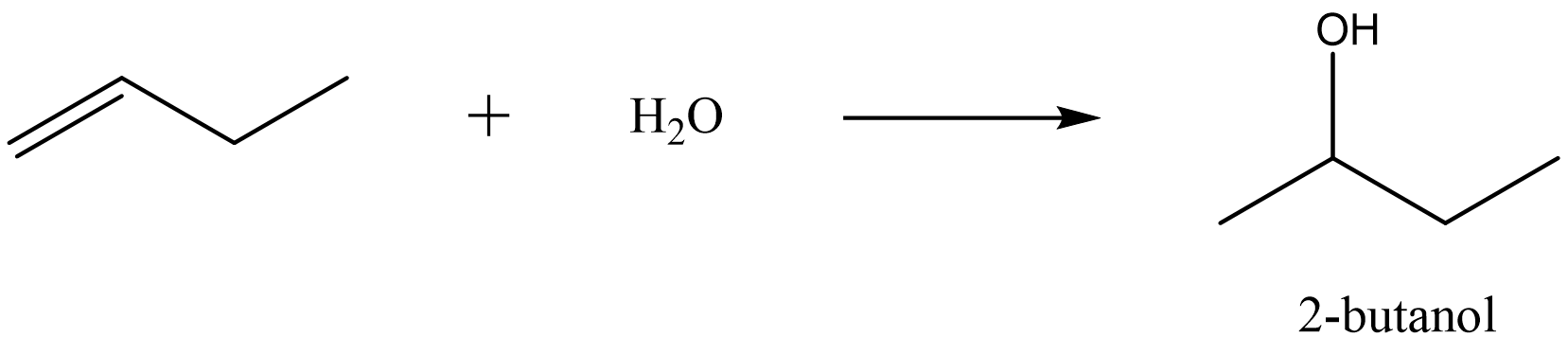
Note that the first carbon in 1-butene started with two hydrogen atoms and the second carbon started with one hydrogen. Therefore, the hydrogen from water adds to the first carbon and the \(\ce{-OH}\) group adds to the second carbon.
Elimination Reactions
An elimination reaction involves the removal of adjacent atoms from a molecule. This results in the formation of a multiple bond and the release of a small molecule, so they are called elimination reactions. They have the general form:
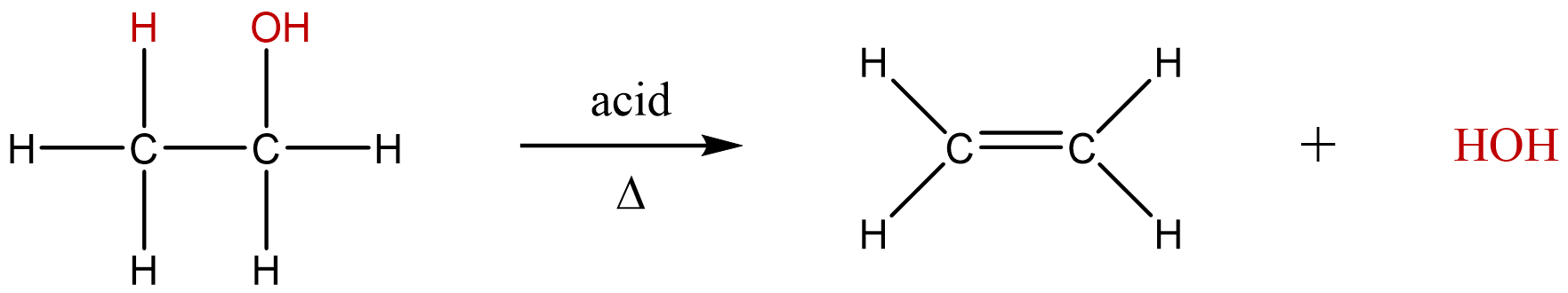
A typical example is the conversion of ethyl chloride to ethylene:
\[CH_3CH_2Cl \rightarrow CH_2=CH_2 + HCl \]
Much of the approximately 26 million tons of ethylene produced per year in the United States is used to synthesize plastics, such as polyethylene. In the above reaction, the A–B molecule eliminated is HCl, whose components are eliminated as H+ from the carbon atom on the left and Cl− from the carbon on the right. When an acid is produced, as occurs here, the reaction is generally carried out in the presence of a base (such as NaOH) to neutralize the acid. Other elimination reactions will produce H2, X2 (where X = halogen), or H2O. These reactions are often referred to by more descriptive terms such as dehydration (removing water), dehydrogenation (removing hydrogen), or dehalogenation (removing a halogen).
Polymerization
Polymers are very different than the other kinds of organic molecules that you have seen so far. Whereas other compounds are of relatively low molar mass, polymers are giant molecules of very high molar mass. Polymers are the primary components of all sorts of plastics and related compounds. A polymer is a large molecule formed of many smaller molecules covalently bonded to one another in a repeating pattern. The small molecules that make up the polymer are called monomers. Polymers are generally formed by either addition or condensation reactions (discussed later in the chapter). Teflon (see figure below) is a non-reactive, non-stick coating used on cookware as well as in containers and pipes for reactive or corrosive chemicals.
Example \(\PageIndex{1}\)
omplete each equation.
-
(CH3) 2C=CH2 + Br2 →
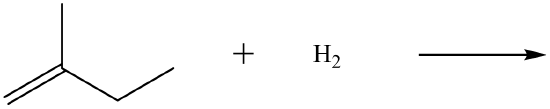
-
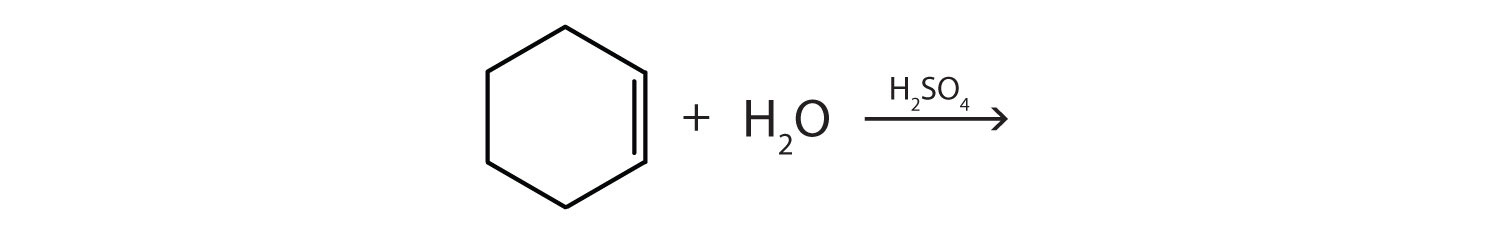
Solution
- (CH3)2CBrCH2Br
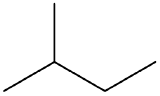
-
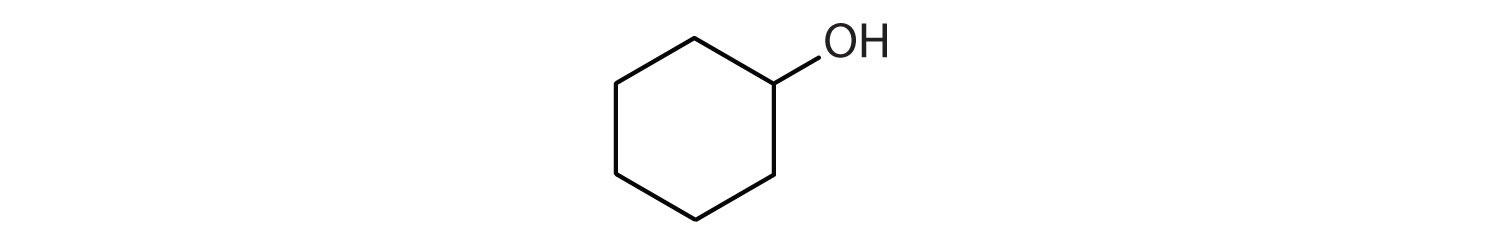
Exercise \(\PageIndex{1}\)
Complete each equation.
KEY TAKEAWAYS
- Alkenes undergo addition reactions, adding such substances as hydrogen, bromine, and water across the carbon-to-carbon double bond.
- Elimination reactions remove groups to form alkenes.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)