5.1: Organic Redox Reactions
- Page ID
- 340358
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify oxidation-reduction reactions with organic compounds.
Oxidation and reduction (redox) reactions are essential to energy production and transfer in living systems. To understand these electron-transfer reactions, chemists separate them into two parts: one part focuses on the loss of electrons, and one part focuses on the gain of electrons. The loss of electrons is called oxidation. The gain of electrons is called reduction. Because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. As such, electron-transfer reactions are also called oxidation-reduction reactions, or simply redox reactions. The atom that loses electrons is oxidized, and the atom that gains electrons is reduced. Also, because we can think of the species being oxidized as causing the reduction, the species being oxidized is called the reducing agent, and the species being reduced is called the oxidizing agent.
Redox reactions are of central importance in organic chemistry and biochemistry. The burning of fuels that provides the energy to maintain our civilization and the metabolism of foods that furnish the energy that keeps us alive both involve redox reactions.

All combustion reactions are also redox reactions. A typical combustion reaction is the burning of methane, the principal component of natural gas (Figure \(\PageIndex{1}\)).
\[\ce{CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O }\label{Eq1}\]
In respiration, the biochemical process by which the oxygen we inhale in air oxidizes foodstuffs to carbon dioxide and water, redox reactions provide energy to living cells. A typical respiratory reaction is the oxidation of glucose (\(\ce{C6H12O6}\)), a simple sugar:
\[\ce{C_6H_{12}O_6 + 6O_2 \rightarrow 6CO_2 + 6H_2O} \label{Eq2}\]
Functional Group Oxidation
Redox reactions involving organic molecules are characterized by the addition or removal of oxygen and/or hydrogen. Organic chemists use a variety of redox reactions. For example, potassium dichromate (\(\ce{K2Cr2O7}\)) is a common oxidizing agent that can be used to oxidize alcohols (symbolized by the general formula ROH). The product of the reaction depends on the location of the OH functional group in the alcohol molecule, the relative proportions of alcohol and the dichromate ion, and reaction conditions such as temperature. If the OH group is attached to a terminal carbon atom (primary alcohol) and the product is distilled off as it forms, the product is an aldehyde, which has a terminal carbonyl group (C=O) and is often written as RCHO. One example is the reaction used by the Breathalyzer to detect ethyl alcohol (\(\ce{CH3CH2OH}\)) in a person’s breath:
\[\ce{3CH_3CH_2OH + Cr_2O_7^{2−} + 8H^+ \rightarrow 3CH_3CHO + 2Cr^{3+} + 7H_2O } \label{Eq3}\]
If the product acetaldehyde (\(\ce{CH3CHO}\)) is not removed as it forms, it is further oxidized to acetic acid (\(\ce{CH3COOH}\)). In this case, the overall reaction is as follows:
\[\ce{3CH_3CH_2OH + 2Cr_2O_7^{2−} + 16H^+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O } \label{Eq4}\]
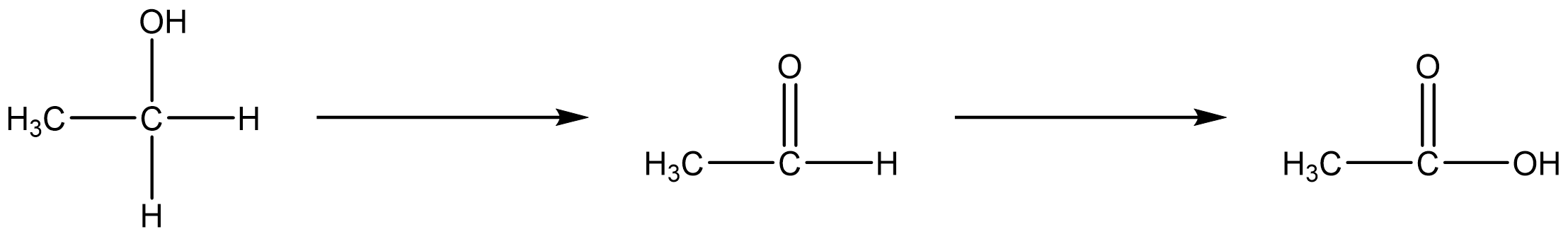
In this reaction, the chromium atom is reduced because it went from \(Cr_2O_7^{2−}\) to \(Cr^{3+}\). On the other hand, the carbon atom in ethanol is oxidized. In the oxidation of ethyl alcohol (CH3CH2OH, a.k.a. ethanol) to form acetaldehayde (CH3CHO, a.k.a. ethanal), the number of bonds to oxygen has increased and the number of hydrogen atoms has decreased from six to four. Either or both of these indicate that an oxidation has occurred.
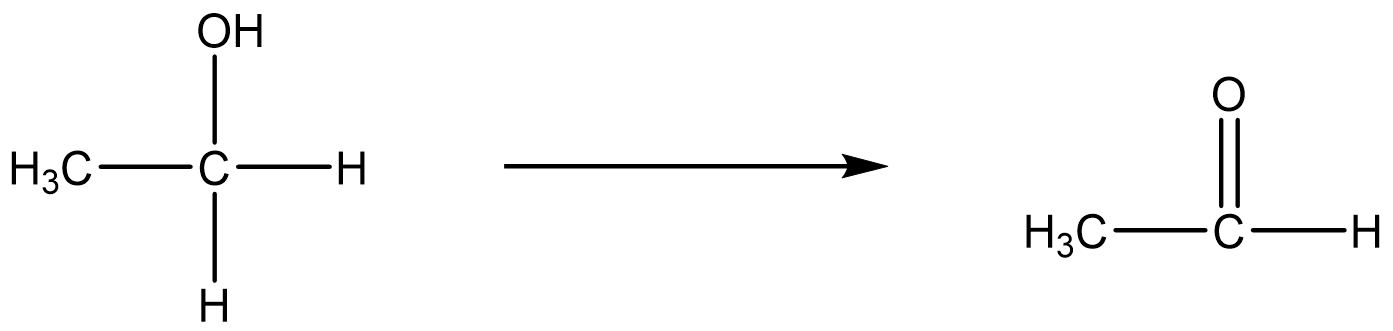
In the oxidation of acetaldehyde to acetic acid (a.k.a. ethanoic acid), the carbon atom that gained an additional oxygen is the element oxidized.
When the alcohol represents a secondary alcohol, the oxidation will produce a ketone (the formulas of ketones are often written as RCOR, and the carbon–oxygen bond is a double bond). The simplest ketone is derived from the oxidation of 2-propanol (\(\ce{CH3CHOHCH3}\)). It is the common solvent acetone [\(\ce{(CH3)2CO}\)], which is used in varnishes, lacquers, rubber cement, and nail polish remover. Acetone can be formed by the following redox reaction:
\[\ce{3CH_3CHOHCH_3 + Cr_2O_7^{2−} + 8H^+ \rightarrow 3(CH_3)_2CO + 2Cr^{3+} + 7H_2O} \label{Eq5}\]
Tertiary alcohols (R3COH) are resistant to oxidation because the carbon atom that carries the OH group does not have a hydrogen atom attached but is instead bonded to other carbon atoms. The oxidation reactions we have described involve the formation of a carbon-oxygen double bond. Thus, the carbon atom bearing the OH group must be able to release one of its attached atoms to form the double bond. The carbon-hydrogen bonding is easily broken under oxidative conditions, but carbon-carbon bonds are not. Therefore tertiary alcohols are not easily oxidized.
Functional Group Reduction
As we have just seen, aldehydes and ketones can be formed by the oxidation of alcohols. Conversely, aldehydes and ketones can be reduced to alcohols. In the reduction of an organic compound, the number of bonds to oxygen decreases and the number of hydrogen atoms increases. Either or both of these indicate that a reduction has occurred. Reduction of the carbonyl group is important in living organisms. For example, in anaerobic metabolism, in which biochemical processes take place in the absence of oxygen, pyruvic acid (\(\ce{CH3COCOOH}\)) is reduced to lactic acid (\(\ce{CH3CHOHCOOH}\)) in the muscles.
\[\ce{CH_3COCOOH \rightarrow CH_3CHOHCOOH } \label{Eq6}\]
(Pyruvic acid is both a carboxylic acid and a ketone; only the ketone group is reduced.) The buildup of lactic acid during vigorous exercise is responsible in large part for the fatigue that we experience.
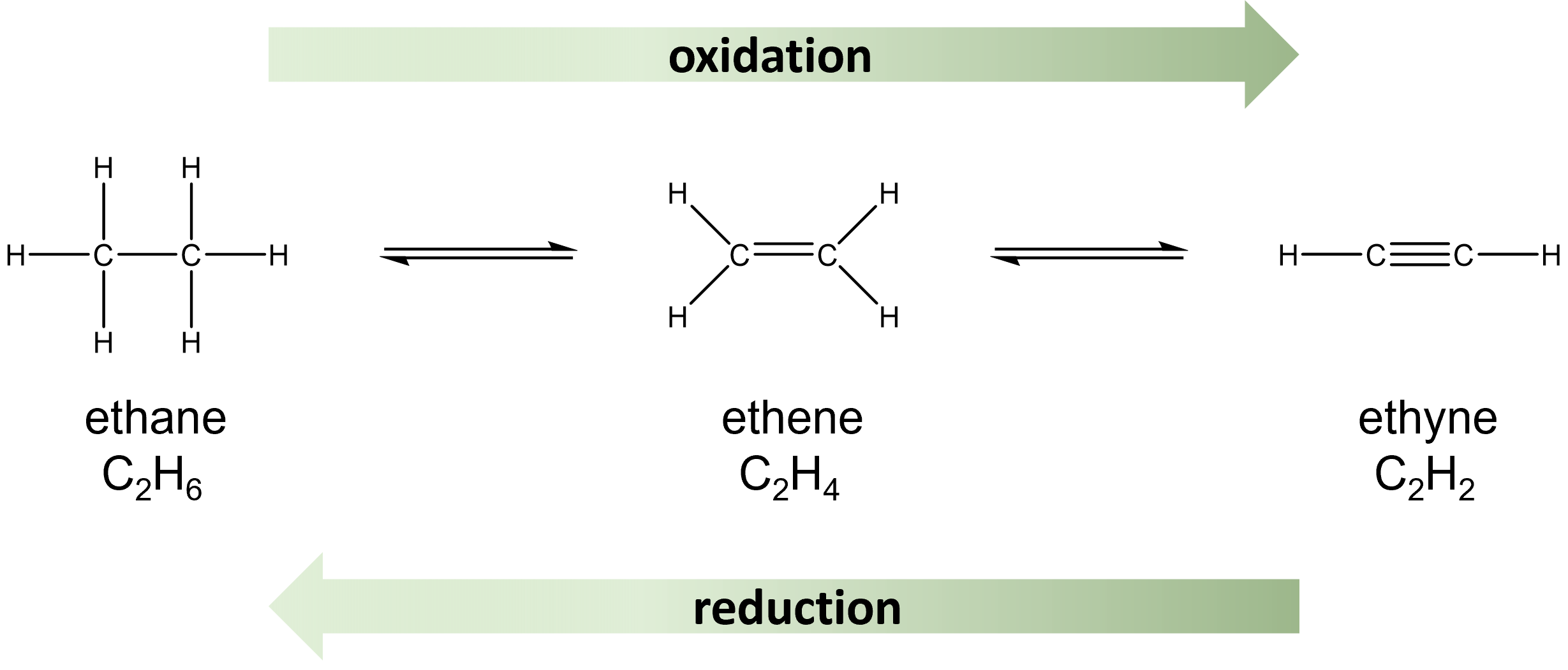
Oxidation and reduction also occur with hydrocarbon molecules. Alkynes can be reduced to alkenes, which can be further reduced to alkanes. The opposite transitions represent the oxidation of alkanes to alkenes, which are further oxidized to alkynes.
Redox Reactions in Food Chemistry
In food chemistry, the substances known as antioxidants are reducing agents. Ascorbic acid (vitamin C; \(\ce{C6H8O6}\)) is thought to retard potentially damaging oxidation of living cells. In the process, it is oxidized to dehydroascorbic acid (\(\ce{C6H6O6}\)). In the stomach, ascorbic acid reduces the nitrite ion (\(\ce{NO_2^{−}}\)) to nitric oxide (\(\ce{NO}\)):
\[\ce{C_6H_8O_6 + 2H^{+} + 2NO_2^{−} \rightarrow C_6H_6O_6 + 2H_2O + 2NO} \label{Eq7}\]
If reaction in Equation \(\ref{Eq7}\) did not occur, nitrite ions from foods would oxidize the iron in hemoglobin, destroying its ability to carry oxygen.
Tocopherol (vitamin E) is also an antioxidant. In the body, vitamin E is thought to act by scavenging harmful by-products of metabolism, such as the highly reactive molecular fragments called free radicals. In foods, vitamin E acts to prevent fats from being oxidized and thus becoming rancid. Vitamin C is also a good antioxidant (Figure \(\PageIndex{5}\)).

Finally, and of greatest importance, green plants carry out the redox reaction that makes possible almost all life on Earth. They do this through a process called photosynthesis, in which carbon dioxide and water are converted to glucose (\(\ce{C6H12O6}\)). The synthesis of glucose requires a variety of proteins called enzymes and a green pigment called chlorophyll that converts sunlight into chemical energy. The overall change that occurs is as follows:
\[\ce{6CO_2 + 6H_2O \rightarrow C_6H_{12}O_6 + 6O_2} \label{Eq8}\]
In this reaction, carbon dioxide is reduced to glucose, and water is oxidized to oxygen gas. Other reactions convert the glucose to more complex carbohydrates, plant proteins, and oils.
Example \(\PageIndex{1}\)
A typical respiratory reaction discussed in the text is the oxidation of glucose (C6H12O6):
C6H12O6 + 6O2 → 6CO2 + 6H2O
Is this a redox reaction? If so, what are the oxidizing and reducing agents?
Solution
Yes; oxidizing agent: O2; reducing agent: C6H12O6
Exercise \(\PageIndex{1}\)
What alcohol is produced in the reduction of propanal (CH3CH2CHO)?
Summary
- Redox reactions are common in organic and biological chemistry, including the combustion of organic chemicals, respiration, and photosynthesis.
- Redox reactions involving organic compounds are characterized by the addition or removal of atoms/bonds:
- oxidation - increase in oxygen and/or decrease in hydrogen
- reduction - decrease in oxygen and/or increase in hydrogen
- Primary alcohols are oxidized to form aldehydes, which are further oxidized to form carboxylic acids.
- Secondary alcohols are oxidized to form ketones.
- Tertiary alcohols are not readily oxidized.

