3.2: Alcohols
- Page ID
- 338691
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify the general structure for an alcohol.
- Explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses.
- Explain why alcohols of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble.
- Identify the structural feature that classifies alcohols as primary, secondary, or tertiary.
- Name alcohols with both common names and IUPAC names.
An alcohol is an organic compound with a hydroxyl (—OH) functional group on an aliphatic carbon atom. Because the hydroxyl (not to be confused with hydroxide) is the functional group of all alcohols, we often represent alcohols by the general formula R—OH, where R is an alkyl group. If the molecule contains two hydroxyl groups, the compound is referred to as a diol. If the alcohol has three hydroxyl groups, the molecule is called a triol. When multiple alcohol functional groups are present, the compound is known as a polyol.
Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates. Methanol (CH3OH) and ethanol (CH3CH2OH) are the first two members of the homologous series of alcohols.
Properties of Alcohols
Alcohols can be considered derivatives of water (H2O; also written as HOH).

Figure \(\PageIndex{1}\): Structural formula of water (left) and an alcohol (right).
Like the H–O–H bond in water, the R–O–H bond is bent, and alcohol molecules are polar. This relationship is particularly apparent in small molecules and reflected in the physical and chemical properties of alcohols with low molar mass. Replacing a hydrogen atom from an alkane with a hydroxyl group allows the molecules to associate through hydrogen bonding (Figure \(\PageIndex{2}\)).
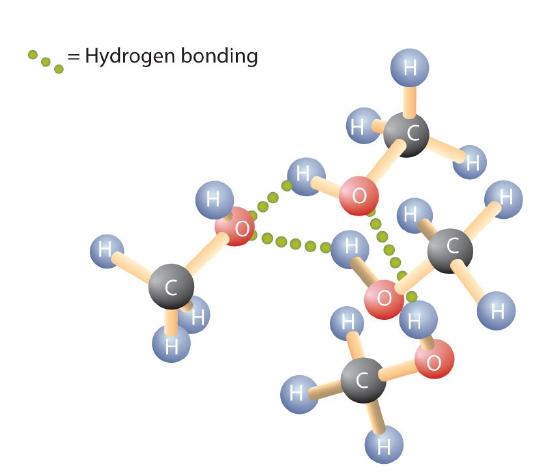
Recall that physical properties are determined to a large extent by the type of intermolecular forces. Table \(\PageIndex{1}\) lists the molar masses and the boiling points of some common compounds. The table shows that substances with similar molar masses can have quite different boiling points.
| Formula | Name | Molar Mass | Boiling Point (oC) |
|---|---|---|---|
| CH4 | methane | 16 | –164 |
| HOH | water | 18 | 100 |
| C2H6 | ethane | 30 | –89 |
| CH3OH | methanol | 32 | 65 |
| C3H8 | propane | 44 | –42 |
| CH3CH2OH | ethanol | 46 | 78 |
| C4H10 | butane | 58 | –1 |
| CH3CH2CH2OH | 1-propanol | 60 | 97 |
Alkanes are nonpolar and are thus associated only through relatively weak dispersion forces. Alkanes with one to four carbon atoms are gases at room temperature. In contrast, even methanol (with one carbon atom) is a liquid at room temperature. Hydrogen bonding greatly increases the boiling points of alcohols compared to hydrocarbons of comparable molar mass. The boiling point is a rough measure of the amount of energy necessary to separate a liquid molecule from its nearest neighbors. If the molecules interact through hydrogen bonding, a relatively large quantity of energy must be supplied to break those intermolecular attractions. Only then can the molecule escape from the liquid into the gaseous state.
Alcohols can also engage in hydrogen bonding with water molecules. Thus, whereas the hydrocarbons are insoluble in water, alcohols with one to three carbon atoms are completely soluble. As the length of the chain increases, however, the solubility of alcohols in water decreases; the molecules become more like hydrocarbons and less like water. The alcohol 1-decanol (CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2OH) is essentially insoluble in water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon atoms.
Nomenclature of Alcohols
Alcohols with one to four carbon atoms are frequently called by common names. The common names of alcohols indicate the identity of the alkyl group followed by the word alcohol (Figure \(\PageIndex{3}\).
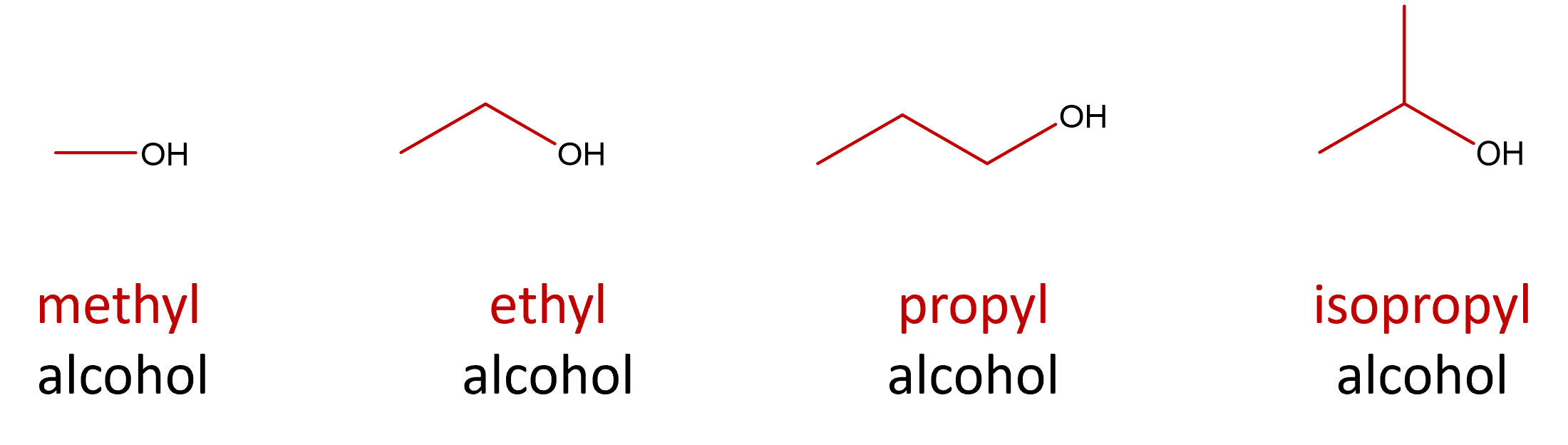
Figure \(\PageIndex{3}\): Structure and common name of some small alcohols.
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named by changing the ending of the parent alkane name to -ol. Here are some basic IUPAC rules for naming alcohols:
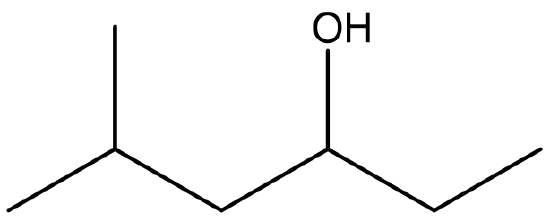
Figure \(\PageIndex{4}\): Skeletal structure of a molecule that will be named as discussing the steps for naming alcohols.
-
The longest continuous chain (LCC) of carbon atoms containing the carbon that the OH group is attached to is considered the parent chain. It is named using the same stem as the alkane having the same number of carbon atoms but the -ane suffix is replaced with -ol to identify it as an alcohol. Thus the parent chain for the molecule in Figure \(\PageIndex{4}\) is a hexanol.
-
The parent chain is numbered to indicate the location of the hydroxyl group. The carbon chain is numbered starting from the end nearest the OH group (even if this causes any substitutents to have a higher number). The number that indicates the position of the functional group is prefixed to the name of the parent hydrocarbon. The locator number may also be shown hyphenated between the stem prefix and -ol suffix. In cyclic alcohols, the carbon atom bearing the OH group is designated C1, but the 1 is not used in the name. The number should always be shown in the acyclic molecules. This parent chain represents 3-hexanol or hexan-3-ol.
-
Substituents are named and numbered as in alkanes. These groups are listed in alphabetical order prior to the parent name. According to these rules, the IUPAC name for the molecule shown in Figure \(\PageIndex{4}\) can be shown as 5-methyl-3-hexanol or 5-methylhexan-3-ol.
Example \(\PageIndex{1}\)
Give the IUPAC name for each compound.
-
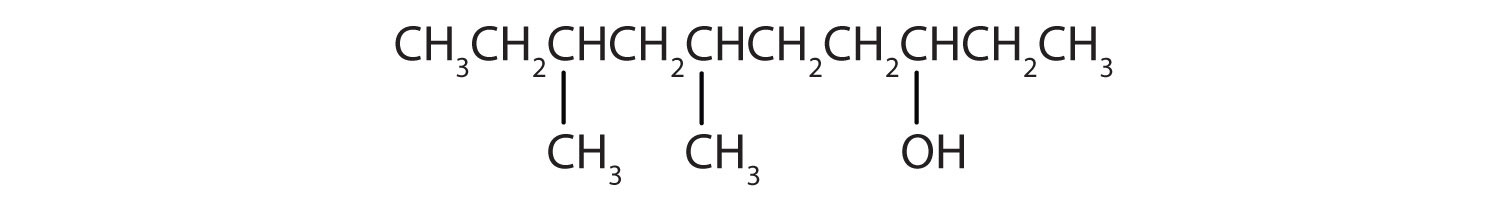
-
CH2CH2CH2CH2CH2OH
Solution
-
Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2). The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl (CH3) groups at the sixth and eighth positions (rule 3). The name is 6,8-dimethyl-3-decanol (not 3,5-dimethyl-8-decanol).
-
Five carbon atoms in the LCC make the compound a derivative of pentane. The OH group is on the first carbon and there are no substituents. This gives the name 1-pentanol.
Exercise \(\PageIndex{1}\)
Give the IUPAC name for each compound.
Example \(\PageIndex{2}\)
Draw the structure for each compound.
-
2-hexanol
-
3-methyl-2-pentanol
Solution
-
The ending -ol indicates an alcohol (the OH functional group), and the hex- stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms: –C–C–C–C–C–C–. The 2 indicates that the OH group is attached to the second carbon atom.
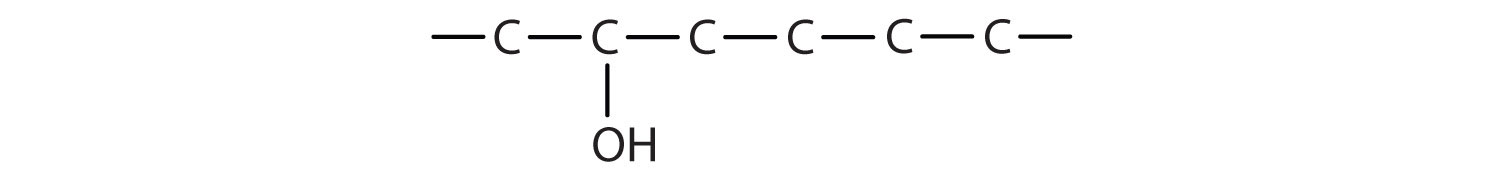
Finally, we add enough hydrogen atoms to give each carbon atom four bonds.
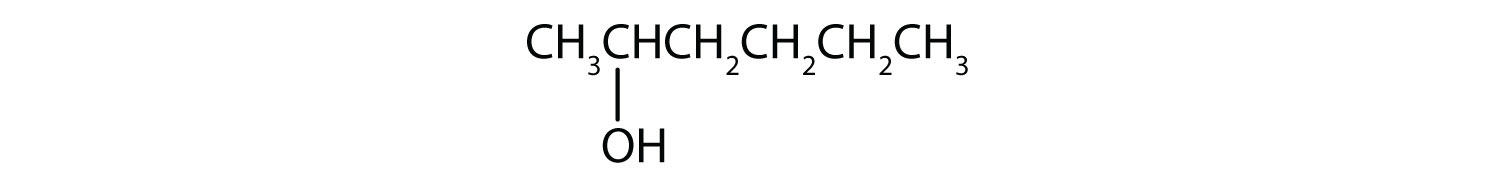
-
The ending -ol indicates an OH functional group, and the pent- stem tells us that there are five carbon atoms in the LCC. We start by drawing a chain of five carbon atoms: –C–C–C–C–C–. The numbers indicate that there is a methyl (CH3) group on the third carbon atom and an OH group on the second carbon atom.
Exercise \(\PageIndex{2}\)
Draw the structure for each compound.
-
3-heptanol
-
2-methyl-3-hexanol
Classification of Alcohols
Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. Alcohols can be grouped into three classes on this basis.
- A primary (1°) alcohol is one in which the carbon atom (in red) with the OH group is attached to one other carbon atom (in blue). Its general formula is RCH2OH.
- A secondary (2°) alcohol is one in which the carbon atom (in red) with the OH group is attached to two other carbon atoms (in blue). Its general formula is R2CHOH.
- A tertiary (3°) alcohol is one in which the carbon atom (in red) with the OH group is attached to three other carbon atoms (in blue). Its general formula is R3COH.
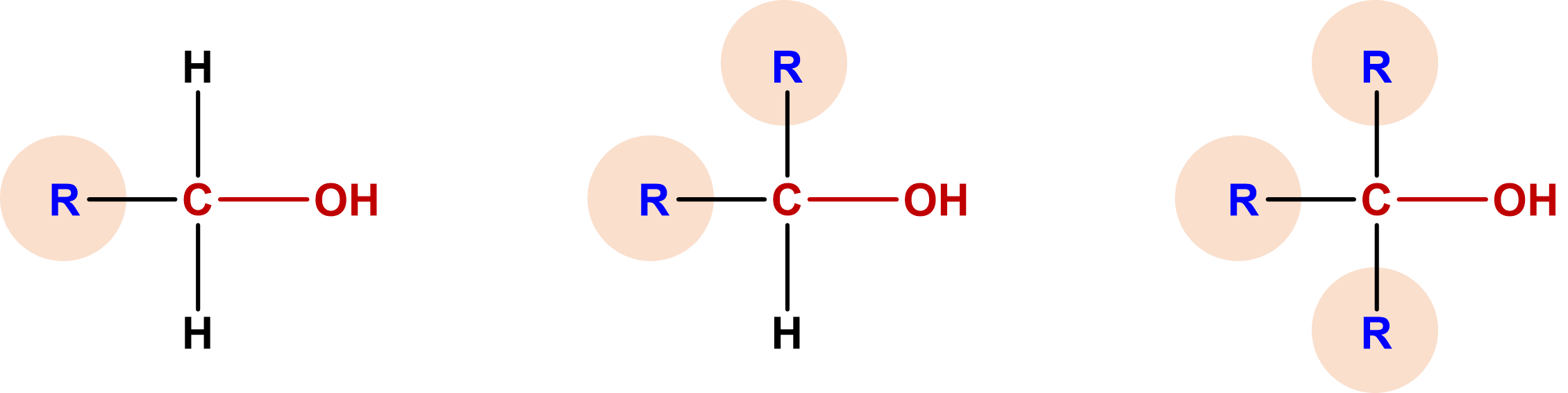
Figure \(\PageIndex{5}\): Classification of Alcohols. Structures of primary (left), secondary (middle), and tertiary (right) alcohols. *R represents the rest of the molecule and can be an acyclic or cyclic group of carbon atoms.
Table \(\PageIndex{2}\) names and classifications of some of the simpler alcohols. Some of the common names reflect a compound’s classification as secondary (sec–) or tertiary (tert–). These designations are not used in the IUPAC nomenclature system for alcohols. Note that there are four butyl alcohols in the table, corresponding to the four butyl groups: the butyl group (—CH2CH2CH2CH3) discussed before, and three others:
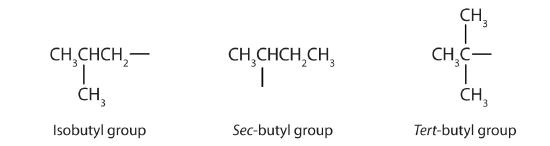
Figure \(\PageIndex{6}\): Common names of butyl substituents.
| Condensed Structural Formula | Class of Alcohol | Common Name | IUPAC Name |
|---|---|---|---|
| CH3OH | — | methyl alcohol | methanol |
| CH3CH2OH | primary | ethyl alcohol | ethanol |
| CH3CH2CH2OH | primary | propyl alcohol | 1-propanol |
| (CH3)2CHOH | secondary | isopropyl alcohol | 2-propanol |
| CH3CH2CH2CH2OH | primary | butyl alcohol | 1-butanol |
| CH3CH2CHOHCH3 | secondary | sec-butyl alcohol | 2-butanol |
| (CH3)2CHCH2OH | primary | isobutyl alcohol | 2-methyl-1-propanol |
| (CH3)3COH | tertiary | tert-butyl alcohol | 2-methyl-2-propanol |
 |
secondary | cyclohexyl alcohol | cyclohexanol |
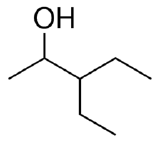
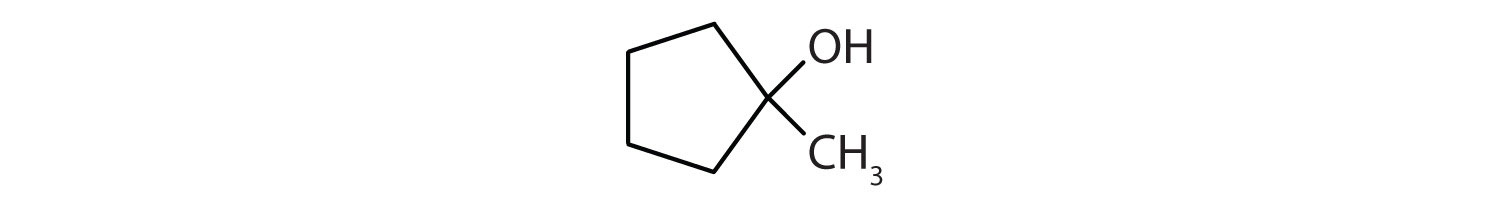
Example \(\PageIndex{3}\)
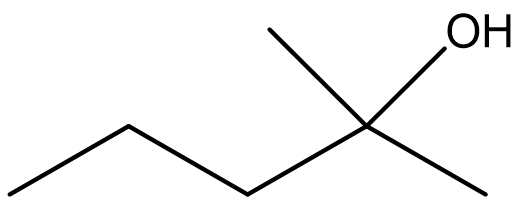
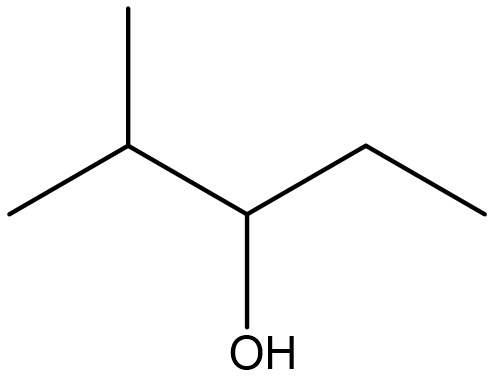
Identify both of the the following as a primary, secondary, or tertiary alcohol.
Solution
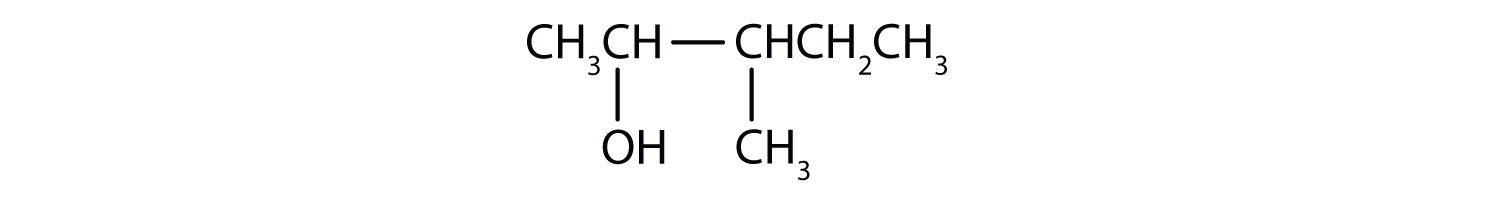
- Secondary. First locate the functional group and identify the carbon that it is bonded to. Then determine how many carbon atoms are directly bonded to it. In this molecule, the functional group is bonded to C3. Since C3 is bonded to C2 and C4, this represents a secondary alcohol.
- Primary. After locating the functional group and the carbon that it is bonded to, it appears that only one additional carbon is attached. Therefore, this represents a primary alcohol.
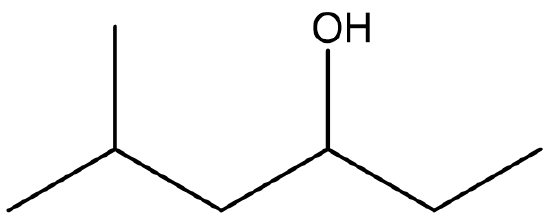
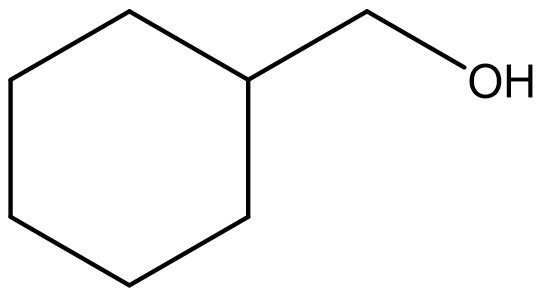
Exercise \(\PageIndex{3}\)
Identify both of the the following as a primary, secondary, or tertiary alcohol.
Summary
- Alcohols have higher boiling points than do ethers and alkanes of similar molar masses because the OH group allows alcohol molecules to engage in hydrogen bonding. Alcohols of four or fewer carbon atoms are soluble in water because the alcohol molecules engage in hydrogen bonding with water molecules; comparable alkane molecules cannot engage in hydrogen bonding.
- IUPAC nomenclature of alcohols: location and identity of substituents + parent prefix (with location of functional group) + ol suffix
- Common name of alcohols: identity of alkyl group + alcohol