15.9: Overview of Lipid Catabolism
- Page ID
- 306693
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- To describe the role of acetyl-CoA in metabolism.
A metabolic pathway is a series of biochemical reactions by which an organism converts a given reactant to a specific end product. There are specific metabolic pathways—which are different for carbohydrates, triglycerides, and proteins—that break down the products of stage I of catabolism (monosaccharides, fatty acids, and amino acids) to produce a common end product, acetyl-coenzyme A (acetyl-CoA) in stage II of catabolism.
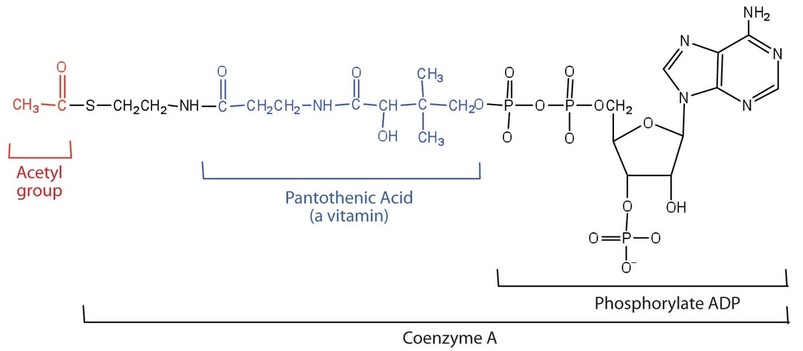
Acetyl-CoA is shown in Figure \(\PageIndex{1}\). The acetyl unit, derived (as we will see) from the breakdown of carbohydrates, lipids, and proteins, is attached to coenzyme A, making the acetyl unit more reactive. Acetyl-CoA is used in a myriad of biochemical pathways. For example, it may be used as the starting material for the biosynthesis of lipids (such as triglycerides, phospholipids, or cholesterol and other steroids). Most importantly for energy generation, it may enter the citric acid cycle and be oxidized to produce energy, if energy is needed and oxygen is available. The various fates or uses of acetyl-CoA are summarized in Figure \(\PageIndex{1}\).

Glycolysis
Glycolysis is the catabolic process in which glucose is converted into pyruvate via ten enzymatic steps. There are three regulatory steps, each of which is highly regulated that are separated into two phases:
- the "priming phase" because it requires an input of energy in the form of 2 ATPs per glucose molecule and
- the "pay off phase" because energy is released in the form of 4 ATPs, 2 per glyceraldehyde molecule.
The end result of Glycolysis is two new pyruvate molecules which can then be fed into the Citric Acid cycle (also known as the Kreb's Cycle) if oxygen is present, or can be reduced to lactate or ethanol in the absence of of oxygen using a process known as fermentation.
Glycolysis occurs within almost all living cells and is the primary source of Acetyl-CoA, which is the molecule responsible for the majority of energy output under aerobic conditions. The structures of Glycolysis intermediates can be found in Figure \(\PageIndex{3}\).
Phase 1: The "Priming Step"
The first phase of Glycolysis requires an input of energy in the form of ATP (adenosine triphosphate).
- alpha-D-Glucose is phosphorolated at the 6 carbon by ATP via the enzyme Hexokinase (Class: Transferase) to yield alpha-D-Glucose-6-phosphate (G-6-P). This is a regulatory step which is negatively regulated by the presence of glucose-6-phosphate.
- alpha-D-Glucose-6-phosphate is then converted into D-Fructose-6-phosphate (F-6-P) by Phosphoglucoisomerase (Class: Isomerase)
- D-Fructose-6-phosphate is once again phosphorolated this time at the 1 carbon position by ATP via the enzyme Phosphofructokinase (Class: Transferase) to yield D-Fructose-1,6-bisphosphate (FBP). This is the committed step of glycolysis because of its large \(\Delta G\) value.
- D-Fructose-1,6-bisphosphate is then cleaved into two, three carbon molecules; Dihydroxyacetone phosphate (DHAP) and D-Glyceraldehyde-3-phosphate (G-3-P) by the enzyme Fructose bisphosphate aldolase (Class: Lyase)
- Because the next portion of Glycolysis requires the molecule D-Glyceraldehyde-3-phosphate to continue Dihydroxyacetone phosphate is converted into D-Glyceraldehyde-3-phosphate by the enzyme Triose phosphate isomerase (Class: Isomerase)
Phase 2: The "Pay Off Step"
The second phase of Glycolysis where 4 molecules of ATP are produced per molecule of glucose. Enzymes appear in red:
- D-Glyceraldehyde-3-phosphate is phosphorolated at the 1 carbon by the enzyme Glyceraldehyde-3-phosphate dehodrogenase to yield the high energy molecule 1,3-Bisphosphoglycerate (BPG)
- ADP is then phosphorolated at the expense of 1,3-Bisphosphoglycerate by the enzyme Phosphoglycerate kinase (Class: Transferase) to yield ATP and 3-Phosphoglycerate (3-PG)
- 3-Phosphoglycerate is then converted into 2-Phosphoglycerate by Phosphoglycerate mutase in preparation to yield another high energy molecule
- 2-Phosphoglycerate is then converted to phosphoenolpyruvate (PEP) by Enolase. H2O, potassium, and magnesium are all released as a result.
- ADP is once again phosphorolated, this time at the expense of PEP by the enzyme pyruvate kinase to yield another molecule of ATP and and pyruvate. This step is regulated by the energy in the cell. The higher the energy of the cell the more inhibited pyruvate kinase becomes. Indicators of high energy levels within the cell are high concentrations of ATP, Acetyl-CoA, Alanine, and cAMP.
Because Glucose is split to yield two molecules of D-Glyceraldehyde-3-phosphate, each step in the "Pay Off" phase occurs twice per molecule of glucose.
Beta-Oxidation
The best source of energy for eukaryotic organisms are fats. Glucose offers a ratio 6.3 moles of ATP per carbon while saturated fatty acids offer 8.1 ATP per carbon. Also the complete oxidation of fats yields enormous amounts of water for those organisms that do not have adequate access to drinkable water. Camels and killer whales are good example of this, they obtain their water requirements from the complete oxidation of fats.
There are four distinct stages in the oxidation of fatty acids. Fatty acid degradation takes place within the mitochondria and requires the help of several different enzymes. In order for fatty acids to enter the mitochondria the assistance of two carrier proteins is required, Carnitine acyltransferase I and II. It is also interesting to note the similarities between the four steps of beta-oxidation and the later four steps of the TCA cycle.
Entry into Beta-oxidation
Most fats stored in eukaryotic organisms are stored as triglycerides as seen below. In order to enter into beta-oxidation bonds must be broken usually with the use of a Lipase. The end result of these broken bonds are a glycerol molecule and three fatty acids in the case of triglycerides. Other lipids are capable of being degraded as well.

Activation Step
- Once the triglycerides are broken down into glycerol and fatty acids they must be activated before they can enter into the mitochondria and proceed on with beta-oxidation. This is done by Acyl-CoA synthetase to yield fatty acyl-CoA.
- After the fatty acid has been acylated it is now ready to enter into the mitochondria.
- There are two carrier proteins (Carnitine acyltransferase I and II), one located on the outer membrane and one on the inner membrane of the mitochondria. Both are required for entry of the Acyl-CoA into the mitochondria.
- Once inside the mitochondria the fatty acyl-CoA can enter into beta-oxidation.
Oxidation Step
A fatty acyl-CoA is oxidized by Acyl-CoA dehydrogenase to yield a trans alkene. This is done with the aid of an [FAD] prosthetic group.
Oxidation Step
The alcohol of the hydroxyacly-CoA is then oxidized by NAD+ to a carbonyl with the help of Hydroxyacyl-CoA dehydrogenase. NAD+ is used to oxidize the alcohol rather then [FAD] because NAD+ is capable of the alcohol while [FAD] is not.
Cleavage
Finally acetyl-CoA is cleaved off with the help of Thiolase to yield an Acyl-CoA that is two carbons shorter than before. The cleaved acetyl-CoA can then enter into the TCA and ETC because it is already within the mitochondria.
Summary
Acetyl-CoA is formed from the breakdown of carbohydrates, lipids, and proteins. It is used in many biochemical pathways.
References
- Garrett, H., Reginald and Charles Grisham. Biochemistry. Boston: Twayne Publishers, 2008.
- Raven, Peter. Biology. Boston: Twayne Publishers, 2005.
Concept Review Exercises
- What is a metabolic pathway?
- What vitamin is required to make coenzyme A?
- What is the net yield of Glycolysis as far as ATP?
- Name the enzymes that are key regulatory sites in Glycolysis.
- Why are the enzymes in the previous question targets for regulation?
- Why is the priming phase necessary?
- Draw the entire pathway for glycolysis including enzymes, reactants and products for each step.
- Where does beta-oxidation occur?
- What is the average net yield of ATP per carbon?
- Where exactly is water formed during the process of fatty acid degradation? (Hint: H2O is formed when when the one of the products of beta-oxidation is passed through another of the metabolic pathways)
- During the process of beta-oxidation, why is it that [FAD] is used to oxidize an alkane to an alkene while NAD+ is used to oxidize an alchol to a carbonyl
- Draw out the entire process of the degradation of a triglyceride, include enzymes and products and reactants for each step.
Answers
- A metabolic pathway is a series of biochemical reactions by which an organism converts a given reactant to a specific end product.
- pantothenic acid
Contributors and Attributions
- Darik Benson, (University California Davis)

