6: Acid-Base and Donor-Acceptor Chemistry
- Page ID
- 326199
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In a very real sense, we can make an acid anything we wish. The differences between the various acid-base concepts are not concerned with which is 'right', but which is most convenient to use in a particular situation.
James E. Huheey, Ellen A. Keiter, and Richard L. Keiter
The concept of acids and bases is often associated with the movement of hydrogen ions from one molecule or ion to another. However, a host of acid base concepts have been developed to help chemists organize and make sense of a wide range of reactions (Table 6.1).
| Definition | Theoretical paradigm and notable features. | Acid | Base | Illustrative sample reactions |
|---|---|---|---|---|
|
Arrhenius (1894) |
Interested in what the substance does to the state of an aqueous solution. In particular it assesses proton donation to & removal from water using [H3O+] as a proxy. Readily accommodates the pH concept as a measure of the state of a solution |
Increases [H3O+] |
Decreases [H3O+] |
\(\underset{acid}{HCl}~+~H_2O~\rightarrow~H_3O^+~+~Cl^-\) \(\underset{base}{NH_3}~+~H_2O~\rightarrow~NH_4^+~+~OH^-\) |
|
Brønsted-Lowry (1923) |
Envisions acid-base reactivity in terms of the transfer of a H+ from one substance to another. Allows for conjugate acids and bases and solvent autoionization. |
Donates H+ |
Accepts H+ |
\(\underset{acid}{HCl}~+~\underset{base}{NH_3}~\rightarrow~\underset{conj.~acid}{NH_4^+}~+~\underset{conj.~base}{Cl^-}\) \(\underset{acid}{HOAc}~+~\underset{base}{NH_3}~\rightarrow~\underset{conj.~acid}{NH_4^+}~+~\underset{conj.~base}{OAc^-}\) \(\underset{amphoteric}{2~H_2O}~\rightarrow~\underset{conj.~acid}{H_3O^+}~+~\underset{conj.~base}{OH^-}\) |
|
Lux-Flood (1939-~47) |
Describes reactions involving oxides and oxyanions in terms of the transfer of oxide ion (O2-). Mainly used in geochemistry, although it also can be used to describe some redox reactions. | Oxide acceptor | Oxide donor |
\(\underset{acid}{SiO_2}~+~\underset{base}{CaO}~\rightarrow~CaSiO_3\) \(\underset{base}{H_2O}~+~\underset{acid}{CO}~\rightarrow~H_2~+~CO_2\) |
| Solvent System |
Applies aspects of the Arrhenius, Brønsted-Lowry, and Lux-Flood acid base concepts to solvent cation & anion formation in a generalized reaction. Can be used to describe solution chemistry in nonaqueous solvent systems like BrF3. |
Is a solvent cation or increases the solvent cation concentration, often by receiving a lone pair bearing group |
Is a solvent anion or increases the solvent anion concentration, often by donating a lone pair bearing group |
\(\underset{acid}{SbF_5}~+~\underset{base}{BrF_3}~\rightarrow~\underset{conj.~base}{SbF_6^-}~+~\underset{conj.~acid}{BrF_2^+}\) \(\underset{amphoteric}{2~BrF_3}~\rightarrow~\underset{conj.~acid}{BrF_2^+}~+~\underset{conj.~base}{BrF_4^-}\) |
| Lewis (1923) |
Envisions acid-base reactivity in terms of electron pair donation. Encompasses the Arrhenius, Brønsted-Lowry, Lux-Flood, and Solvent System definitions and readily integrates with molecular orbital descriptions of chemical reactivity in Frontier orbital theory. |
Accepts an electron pair |
Donates an electron pair |
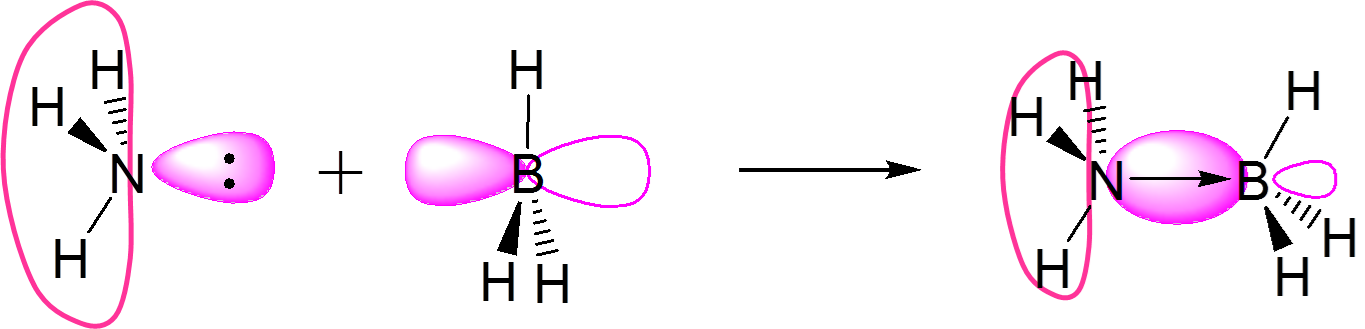
\(\underset{base}{:NH_3}~+~\underset{acid}{BF_3}~\rightarrow~H_3N→BF_3\) |
|
Nucleophile-Electrophile |
Applies the Lewis concept to organic reactivity. Nucleophiles are Lewis bases which tend to react form a bond with Lewis acid sites called electrophilic centers. |
(The electrophile) |
(The nucleophile) Donates an electron pair to form a bond to an electrophile |
\(\underset{base}{Br^-}~+~\underset{acid}{CH_3-Cl}~\rightarrow~Br-CH_3~+~Cl^-\) |
|
Usanovich (1939) |
Extends Lewis theory to include the donation and acceptance of any number of electrons, whether through the formation of an adduct or electron transfer. | Accepts electrons | Donates electrons |
\(\underset{base}{:NH_3}~+~\underset{acid}{BH_3}~\rightarrow~\underset{adduct}{H_3N-BH_3}\) \(\underset{acid}{Fe^{2+}}~+~\underset{base}{Zn^0}~\rightarrow~Fe^0~+~Zn^{2+}\) |
| Frontier Orbital (1960s) | Envisions Lewis Acid-base/Electrophile-nucleophile reactions in terms of the donation and acceptance of electrons between the reactant's frontier orbitals. Specifically, the reaction is envisioned in terms of donation of the base's HOMO electrons into the acid's HOMO level. | Possesses a LUMO capable of forming an occupied bonding MO on mixing with a base's HOMO. | Possesses an electron-bearing HOMO capable of forming a filled bonding MO on mixing with an acid's LUMO. |
base acid adduct |
Some concepts involve defining acids and bases in particular ways that allow for the understanding of particular types of chemical systems. For example, the familiar Arhennius and Brønsted acids and base concepts used in general chemistry help chemists make sense of the behavior of compounds which can transfer H+ ions among themselves, often in aqueous solution. However, the solvent system acid-base concept defines acids and bases in terms of the transfer of a lone-pair bearing group and is particularly useful for conceptualizing the reactivity of main group halides, oxides, and related compounds. Some acid-base definitions seek to encompass an extremely wide range of chemical reactions. For instance, the Lewis acid-base definition encompasses the Arrhenius, Brønsted, and solvent system definitions and has also found wide use in inorganic chemistry owing to the ease with which Lewis acid-base interactions may be described by the Frontier Orbital approach in terms of interacting molecular orbitals on the acid and base.
References
Huheey, J. E.; Keiter, E. A.; Keiter, R. L., Inorganic Chemistry: Principles of Structure and Reactivity. 4th ed.; HarperCollins: New York, NY, 1993, pg. 318.
Contributors and Attributions
- Stephen M. Contakes (Westmont College)
Learning Objectives
- Understand when to apply different acid and base theories
- Identify conjugate acids and bases, and rules for strong & weak acids/bases, in both Brønsted and Lewis acid-base systems
- Describe and rationalize acid/base chemistry of "non-traditional" Brønsted acids.
- Predict favorable and stable compounds using hard-soft acid-base (HSAB) theory.
Thumbnail image is the Lewis acid-base adduct formed between BF3 and NH3.
- 6.1: Arrhenius Model
- The Arrhenius acid-base concept is the "general chemistry" definition of acids and bases. It defines acids and bases in terms of how they affect the concentration of hydronium ions, and by extension hydroxide ions, in aqueous solutions
- 6.2: Brønsted-Lowry Model
- The Brønsted-Lowry acid base concept overcomes the Arrhenius system's inability to describe reactions that take place outside of aqueous solution by moving the focus away from the solution and onto the acid and base themselves.
- 6.3: Lewis Concept and Frontier Orbitals
- The Lewis acid base concept generalizes the Brønsted and solvent system acid base concepts by describing acid-base reactions in terms of the donation and acceptance of an electron pair. Under the Lewis definition Lewis acids are electron pair acceptors and Lewis bases are electron pair donors.
- 6.4: Hard and Soft Acids and Bases
- The hard-soft acid-base principle is a conceptual tool for thinking about patterns of Lewis acid base reactivity. The explanation of the trends in metal distribution, halide salt solubility, and preferred metal coordination patterns is rooted in the observation that Lewis acids and bases could be classified into two groups based on their propensity to form stable compounds with one another.