6.5: Enthalpy – A Modified Energy of Reaction
- Page ID
- 169994
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enthalpy
To avoid having to constantly calculate and compensate for PV work when they are carrying out reactions involving gases, chemists created a modified measure of the energy produced or consumed in a reaction. This measure is called enthalpy and given the symbol H. Enthalpy is defined as follows:
H = E + PV
Here, E is the chemical energy of a substance, and P and V are the pressure and volume of the substance. To calculate a value of H for a substance, we would need to know the value of E. Since it isn’t possible to measure internal energy, we can never calculate or measure the enthalpy of a substance. However, we can measure the change in the internal energy of a substance, so we can also measure changes in enthalpy.
For instance, suppose we wanted to know the change in enthalpy (\(\Delta H)\) when we convert two moles of NO2 into one mole of N2O4. The chemical equation is:
2 NO2(g) --> N2O4(g)
For this reaction, E is -54.72 kJ. What is (\(\Delta H)\)? To find a way to calculate DH, let us start with some algebra, remembering that H = E + PV and (\(\Delta H)\) = Hfinal – Hinitial…
\(\Delta H\) = Hfinal – Hinitial
\(\Delta H\) = (E + PV)final – (E + PV)initial
\(\Delta H\) = (Efinal + PfinalVfinal) – (Einitial + PinitialVinitial)
\(\Delta H\) = (Efinal – Einitial) + (PfinalVfinal – PinitialVinitial)
\(\Delta H\) = \(\Delta E\) + (PfinalVfinal – PinitialVinitial)
The last equation tells us that we can calculate \(\Delta H\) if we can calculate the product P x V before and after the reaction. Since all of the chemicals are gases, we can use PV = nRT, but we still need to know the temperature. \(\Delta E\) is normally measured at 25ºC (298 K), so let’s calculate \(\Delta H\) at 298 K. Making the substitution PV = nRT gives us…
\(\Delta H\) = \(\Delta E\) + (nfinalRT – ninitialRT)
\(\Delta H\) = \(\Delta E\) + RT(nfinal – ninitial)
According to the balanced equation, the reaction converts two moles of gaseous NO2 into one mole of gaseous N2O4, so we can now calculate (\(\Delta H)\):
\(\Delta H\) = -54.72 kJ + (8.314 J/mol·K)(298 K)(1 mol – 2 mol)
\(\Delta H\) = -54.72 kJ + (-2478 J)
\(\Delta H\) = -54.72 kJ + (-2.478 kJ)
\(\Delta H\) = -57.20 kJ
This value of \(\Delta H\) is a combination of two physical effects. First, the internal energy of the chemicals decreases by 54.72 kJ when two moles of NO2 combine to form one mole of N2O4. Second, the value of PV decreases by 2.478 kJ when two moles of NO2 combine to form one mole of N2O4. This makes sense; when the number of moles of gas decreases, the volume or the pressure (or both) must drop.
Why do chemists use change in enthalpy (\(\Delta H)\) more often than (\(\Delta E)\)?
What is the utility of combining internal energy and PV? The answer is that when the pressure is constant, the expression (PfinalVfinal – PinitialVinitial) equals the PV work, but with the opposite sign. Here’s how we can prove it:
If the pressure is constant, Pinitial = Pfinal
So we can just write “P” in place of both Pinitial and Pfinal
Then (PfinalVfinal – PinitialVinitial) becomes (PVfinal – PVinitial)
Factoring out P gives us P(Vfinal – Vinitial)
But Vfinal – Vinitial is an expanded way to write (\(\Delta V)\)
So (PfinalVfinal – PinitialVinitial) = P(\(\Delta V)\)
The PV work is given by the expression wPV = -P(\(\Delta V)\)
So (PfinalVfinal – PinitialVinitial) = -wPV
Earlier, we saw that
\(\Delta H\) = \(\Delta E\) + (PfinalVfinal – PinitialVinitial)
So, after all of this algebra, we reach the following statement
If the pressure is constant, \(\Delta H\) = \(\Delta E\) + (-wPV)
Now, let’s bring back the First Law of Thermodynamics, which tells us that (\(\Delta E)\) = q + w. Substituting this expression for \(\Delta E\) gives us:
If the pressure is constant, \(\Delta H\) = (q + w) + (-wPV)
Simplifying this equation, \(\Delta H\) = q + (w - wPV) where w is all types of work and wPV is specifically PV work, meaning that the difference (w - wPV) is really just any work that is done other than PV work. Substituting (wother) into the equation, we arrive at the following statement:
If the pressure is constant, \(\Delta H\) = q + wother
And, as long as we don’t run our reaction in some sort of machine or battery, etc., then no other work will be done (wother = 0) and the above equation reduced to:
If the pressure is constant, and no work other than PV work is done,
\(\Delta H\) = q
This is why chemists “invented” enthalpy. When the pressure is constant, the \(\Delta H\) for a reaction tells us the amount of heat the reaction will produce or absorb. We no longer have to calculate PV work. Remember that “constant pressure” simply means that the reaction occurs in an open container, so the chemical mixture is free to expand or contract. Since we carry out most reactions under these conditions, chemists normally use \(\Delta H\) instead of \(\Delta E\) to describe the energy given off or absorbed by a reaction.
Let’s put this idea into practice. A while ago, we looked at the reaction 2 NO2(g) --> N2O4(g), and we found that for this reaction, \(\Delta E\) = -54.72 kJ and \(\Delta H\) = -57.20 kJ. What do these numbers tell us?
- If we carry out the reaction in a sealed, rigid container, the amount of heat we get will equal \(\Delta E\): we will get 54.72 kJ of heat.
- If we carry out the reaction in an open container, the amount of heat we get will equal \(\Delta H\): we will get 57.20 kJ of heat.
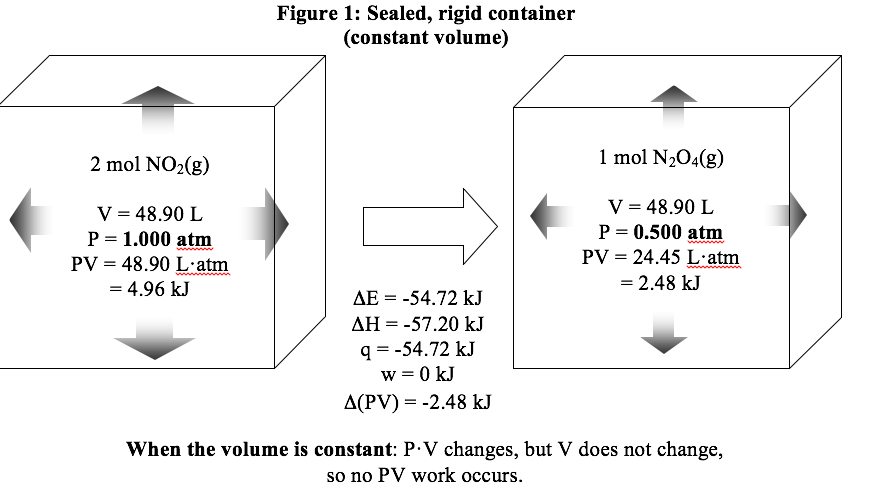
It is important to recognize that the values of \(\Delta E\) and \(\Delta H\) do not depend on whether we carry out the reaction in an open or closed container. For this reaction, \(\Delta H\) is -57.20 kJ even if the reaction occurs in a sealed, rigid container. However, under these conditions, \(\Delta H\) does not equal the heat. In fact, \(\Delta H\) doesn’t really mean anything very useful when we are in a sealed, rigid container. All it tells us is that the quantity (E + PV) is decreasing by 57.20 kJ. Since E itself decreases by 54.72 kJ, the product P·V decreases by 2.48 kJ (we can write this as \(\Delta (PV)\) = -2.48 kJ). In a sealed, rigid container, P·V changes because P changes while V stays constant; since V is constant, there is no PV work. In an open container, P·V changes because V changes while P stays constant; in this case, there is PV work. The following two figures compare the two ways of running the reaction.

And so, if a chemical or physical process is carried out at constant pressure with the only work done caused by expansion or contraction, then the heat flow (\(q_\ce{p}\)) and enthalpy change (\(ΔH\)) for the process are equal.
The heat given off when you operate a Bunsen burner is equal to the enthalpy change of the methane combustion reaction that takes place, since it occurs at the essentially constant pressure of the atmosphere. On the other hand, the heat produced by a reaction measured in a bomb calorimeter is not equal to \(ΔH\) because the closed, constant-volume metal container prevents expansion work from occurring. Chemists usually perform experiments under normal atmospheric conditions, at constant external pressure with \(q = ΔH\), which makes enthalpy the most convenient choice for determining heat.
The following conventions apply when we use \(ΔH\):
- Chemists use a thermochemical equation to represent the changes in both matter and energy. In a thermochemical equation, the enthalpy change of a reaction is shown as a ΔH value following the equation for the reaction. This \(ΔH\) value indicates the amount of heat associated with the reaction involving the number of moles of reactants and products as shown in the chemical equation. For example, consider this equation: \[\ce{H2(g) + 1/2 O2(g) ⟶ H2O (l)} \;\; ΔH=\mathrm{−286\:kJ} \label{5.4.6}\] This equation indicates that when 1 mole of hydrogen gas and 12 mole of oxygen gas at some temperature and pressure change to 1 mole of liquid water at the same temperature and pressure, 286 kJ of heat are released to the surroundings. If the coefficients of the chemical equation are multiplied by some factor, the enthalpy change must be multiplied by that same factor (ΔH is an extensive property).
\[\begin {align*} &\textrm{(two-fold increase in amounts)}\label{5.4.7}\\ &\ce{2H2}(g)+\ce{O2}(g)⟶\ce{2H2O}(l)\hspace{20px}ΔH=\mathrm{2×(−286\:kJ)=−572\:kJ}\\ &\textrm{(two-fold decrease in amounts)}\\ &\frac{1}{2}\ce{H2}(g)+\dfrac{1}{4}\ce{O2}(g)⟶\frac{1}{2}\ce{H2O}(l)\hspace{20px}ΔH=\mathrm{\frac{1}{2}×(−286\:kJ)=−143\:kJ} \end {align*} \]
- The enthalpy change of a reaction depends on the physical state of the reactants and products of the reaction (whether we have gases, liquids, solids, or aqueous solutions), so these must be shown. For example, when 1 mole of hydrogen gas and 1/2 mole of oxygen gas change to 1 mole of liquid water at the same temperature and pressure, 286 kJ of heat are released. If gaseous water forms, only 242 kJ of heat are released.
\[\ce{ H2(g) + 1/2 O2(g) ⟶ H2O(g)} \;\;\; ΔH=\ce{−242\:kJ} \]
- A negative value of an enthalpy change, ΔH, indicates an exothermic reaction; a positive value of ΔH indicates an endothermic reaction. If the direction of a chemical equation is reversed, the arithmetic sign of its ΔH is changed (a process that is endothermic in one direction is exothermic in the opposite direction).
Example \(\PageIndex{1}\): Measurement of an Enthalpy Change
When 0.0500 mol of HCl(aq) reacts with 0.0500 mol of NaOH(aq) to form 0.0500 mol of NaCl(aq), 2.9 kJ of heat are produced. What is ΔH, the enthalpy change, per mole of acid reacting, for the acid-base reaction run under the conditions described ?
\[\ce{HCl (aq) + NaOH(aq) \rightarrow NaCl (aq) + H2O(l)} \nonumber \]
Solution
For the reaction of 0.0500 mol acid (HCl), q = −2.9 kJ. This ratio
\[\mathrm{\dfrac{−2.9 \; kJ}{0.0500\; mol\; HCl}} \nonumber\]
can be used as a conversion factor to find the heat produced when 1 mole of HCl reacts:
\[ΔH =\mathrm{1\; \cancel{mol\; HCl} \times \dfrac{ −2.9\; kJ}{0.0500 \;\cancel{ mol\; HCl}} =−58\; kJ} \nonumber\]
The enthalpy change when 1 mole of HCl reacts is −58 kJ. Since that is the number of moles in the chemical equation, we write the thermochemical equation as:
\[\ce{HCl(aq)+NaOH(aq) \rightarrow NaCl(aq)+H2O(l) \;\;\; ΔH=\mathrm{−58\;kJ} \nonumber\]
Exercise \(\PageIndex{1}\)
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine the enthalpy change per mole of zinc reacting for the reaction:
\[\ce{Zn (s) + 2HCl (aq) \rightarrow ZnCl (aq) + H2 (g)} \nonumber\]
- Answer
-
ΔH = −153 kJ
Be sure to take both stoichiometry and limiting reactants into account when determining the ΔH for a chemical reaction.
Example \(\PageIndex{2}\): Another Example of the Measurement of an Enthalpy Change
A gummy bear contains 2.67 g sucrose, C12H22O11. When it reacts with 7.19 g potassium chlorate, KClO3, 43.7 kJ of heat are produced. Determine the enthalpy change for the reaction
\[\ce{C12H22O11 (aq) + 8 KClO3 (aq) \rightarrow 12 CO2 (g) + 11 H2O (l) + 8KCl (aq)} \nonumber\]
Solution
We have \(\mathrm{2.67\:\cancel{g}×\dfrac{1\:mol}{342.3\:\cancel{g}}=0.00780\:mol\:C_{12}H_{22}O_{11}}\) available, and
\(\mathrm{7.19\:\cancel{g}×\dfrac{1\:mol}{122.5\:\cancel{g}}=0.0587\:mol\:KClO_3}\) available.
Since
\(\mathrm{0.0587\:mol\:KClO_3×\dfrac{1\:mol\:\ce{C12H22O11}}{8\:mol\:KClO_3}=0.00734\:mol\:\ce{C12H22O11}}\)
is needed, C12H22O11 is the excess reactant and KClO3 is the limiting reactant.
The reaction uses 8 mol KClO3, and the conversion factor is \(\mathrm{\dfrac{−43.7\:kJ}{0.0587\:mol\:KClO_3}}\), so we have \(ΔH=\mathrm{8\:mol×\dfrac{−43.7\:kJ}{0.0587\:mol\:KClO_3}=−5960\:kJ}\). The enthalpy change for this reaction is −5960 kJ, and the thermochemical equation is:
\[\ce{C12H22O11 + 8KClO3⟶12CO2 + 11H2O + 8KCl}\hspace{20px}ΔH=\ce{−5960\:kJ} \nonumber\]
Exercise \(\PageIndex{2}\)
When 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of \(\ce{FeCl}_{2(s)}\) and 8.60 kJ of heat is produced. What is the enthalpy change for the reaction when 1 mole of \(\ce{FeCl2(s)}\) is produced?
- Answer
-
ΔH = −338 kJ
Relating \(\Delta H\) to \(\Delta E\)
The formal relationship between \(\Delta H\) and \(\Delta E\) is \(\Delta H\) = \(\Delta E\) + \(\Delta (PV)\). However, we do not normally use this equation, because we do not specify the pressure and volume. As we did on page 11, we assume that any change in volume is due to a change in the number of moles of gases, so we make the substitution PV = nRT. We also assume constant temperature. This allows us to factor out R and T as shown below:
\(\Delta H\) = \(\Delta E\) + \(\Delta (PV)\)
\(\Delta H\) = \(\Delta E\) + (PfinalVfinal – PinitialVinitial)
\(\Delta H\) = \(\Delta E\) + (nfinalRT – ninitialRT)
\(\Delta H\) = \(\Delta E\) + RT(nfinal – ninitial)
\(\Delta H\) = \(\Delta E\) + RT\(\Delta n_{gases}\)
We can use this equation to translate \(\Delta H\) into \(\Delta E\) (and vice versa), regardless of the actual pressure and volume. All we need to know is the temperature (which must be given to us) and the change in the number of moles of gases, which we can get using stoichiometry.
Example \(\PageIndex{3}\): Calculating \(\Delta H\) given \(\Delta E\)
For instance, suppose we know that \(\Delta E\) = -918.2 kJ for the following reaction at 298 K:
4 NH3(g) + 3 O2(g) --> 2 N2(g) + 6 H2O(l)
What is \(\Delta H\) for this reaction at 298 K? We can use the relationship between \(\Delta H\) and \(\Delta E\); all we need to do is determine the change in the number of moles of gases. This reaction converts 7 moles of gaseous reactants (4 moles of NH3 and 3 moles of O2) into 2 moles of gaseous products (the N2), so \(\Delta ngases\) = 2 mol – 7 mol = -5 mol.
\(\Delta H\) = -918.2 kJ + (8.314 J/mol·K)(298 K)(-5 mol)
= -918.2 kJ + (-12388 J)
= -918.2 kJ + (-12.4 kJ)
= -930.6 kJ
Easy enough, eh? Just be sure that you pay attention to units; \(\Delta H\) and \(\Delta E\) are normally given in kJ, but the \(RT\Delta n_{gases}\) term is in joules and must be converted to kJ.

