6.4: Energy and Chemical Change
- Page ID
- 169993
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Potential Energy and Kinetic Energy for a Chemical System
Any collection of matter has two types of energy, kinetic energy and potential energy. These types of energy are defined as follows:
- Kinetic energy is the energy an object has because it is moving.
- Potential energy is the energy an object has because of its position.
For example, a rolling ball has energy because it is moving; it can do work if it hits something. A stationary ball that is high in the air also has energy, it can do work if it is allowed to fall.
In chemistry, we are primarily concerned with thermal energy and chemical energy. Thermal energy is the energy in a hot object; a hot piece of metal has more energy than a cold piece of metal. Thermal energy is due to the motion of the atoms in the object; in a hot object, the atoms are moving rapidly, whereas in a cold object they are moving more slowly. Since this energy is due to motion, thermal energy is a type of kinetic energy.
Chemical energy is the energy that will be released or absorbed when chemicals react with one another. For example, Bunsen burners (and gas applicances) use the reaction between CH4 (methane, the primary constituent of natural gas) and O2 to produce heat:
\[\ce{ CH4 (g) + 2 O2 (g) \rightarrow CO2 (g) + 2 H2O (l)} \nonumber\]
If you mix some CH4 and some oxygen at room temperature, the mixture will remain unchanged indefinitely. However, if you strike a spark, the two gases react violently, producing a great deal of thermal energy. This thermal energy does not “come from nowhere”; it was originally stored in the CH4 and O2 molecules. This stored energy is undetectable to us until the reaction occurs; a mixture of CH4 and O2 looks just like a mixture of He and Ne (which cannot react with one another and have no stored energy). The energy that is stored in a mixture of CH4 and O2 is chemical energy, and it is due to the positions of the atoms. In this reaction, the atoms have lower potential energies when the oxygen atoms are bonded to carbon and hydrogen, rather than to each other, as shown below.
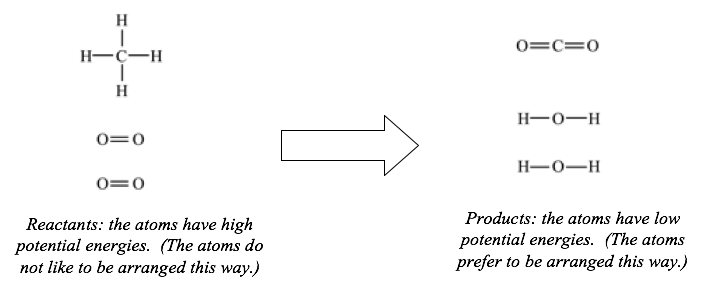
Since chemical energy depends on positions of atoms, chemical energy is a type of potential energy. Chemical energy does not depend on temperature.
Energy transfers and and chemical reactions
Most reactions that we encounter produce thermal energy; the reacting chemicals, and everything around them, become hot. Any reaction that produces thermal energy is called exothermic. In an exothermic reaction, heat seems to come from nowhere. However, no process can simply create energy, because doing so would violate the principle of conservation of energy. Instead, exothermic reactions convert chemical energy into thermal energy. This thermal energy is then transferred from the reaction products to the surroundings. We can illustrate this conversion graphically as shown below.
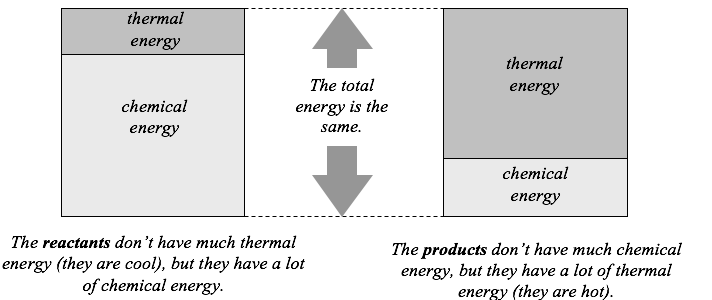
As you can see from the figure, the overall energy change in any reaction is zero; energy is neither created nor destroyed. However, the amount of chemical energy changes in any reaction; the products never contain the same amount of chemical energy as the reactants do. When chemists talk about the “energy of a reaction,” they always mean the change in the chemical energy.
Here is a simple example of how we might measure the energy of a reaction. Suppose that we carry out the reaction in a container that is surrounded by water. After the reaction, we find that the water is hotter; and by measuring the temperature change and the mass, we find that the water has absorbed 50 kJ of heat. From the standpoint of the water, the heat is a positive number, since the water gained energy. We can write
qwater = + 50 kJ
where the subscript “water” means that the water is our system (for the moment).
Now, our real interest is in the chemical reaction. Recall the the First Law of Thermodynamics: In any process, energy can never be created or destroyed; it can only be transferred from one object to another in the form of heat and/or work. Or, stated as a mathematical equation: \(\Delta E = q + w\). The First Law tells us that the heat that went into the water must have come out of the reacting chemicals, so the reacting chemicals must have lost 50 kJ of heat. From the standpoint of the chemicals, the heat is a negative number. We can write:
qchemicals = -50 kJ
We did not allow any work to be done, so wchemicals = 0. Now we can use the First Law of Thermodynamics to calculate the energy change that occurred in the chemicals.
\(\Delta\) Echemicals = qchemicals + wchemicals
= (-50 kJ) + 0 kJ
= -50 kJ
The energy of the chemicals decreased by 50 kJ during the reaction. This \(\Delta E\) is the change in the chemical energy; the reactants had 50 kJ more potential energy than the products did. Note that the value of \(\Delta E\) is negative; \(\Delta E\) is negative for an exothermic reaction.
Some reactions consume thermal energy. In this type of reaction, called an endothermic reaction, the chemicals and their surroundings become colder. Endothermic reactions convert thermal energy into chemical energy. Since the chemical energy cannot be felt or detected, these reactions seem to make energy disappear. For an endothermic reaction, all of the signs we saw above are reversed. For instance, suppose that we carry out a reaction in a container that is surrounded by water, and after the reaction we find that the water has become colder. By measuring the temperature change and the mass, we find that the water has lost 30 kJ of heat. From the standpoint of the water, then, q = -30 kJ. This heat was absorbed by the reacting chemicals, so from the standpoint of the chemicals, q = +30 kJ. By the First Law of Thermodynamics, \(\Delta E\) = +30 kJ for this reaction. The products have 30 kJ more chemical energy than the reactants had. \(\Delta E\) is positive for an endothermic reaction.
Here is a summary of the differences between exothermic and endothermic reactions:
|
In an exothermic reaction: |
In an endothermic reaction: |
|
Everything gets warmer. |
Everything gets colder. |
|
The reaction changes chemical energy into thermal energy. |
The reaction changes thermal energy into chemical energy. |
|
q is positive for the surroundings. |
q is negative for the surroundings. |
|
q is negative for the chemicals. |
q is positive for the chemicals. |
|
\(\Delta E\) is negative. |
\(\Delta E\) is positive. |
Energy and Reaction Stoichiometry
The amount of energy that a reaction produces or absorbs depends on the amounts of chemicals that react. This should seem obvious; for instance, two gallons of gasoline produce twice as much heat as one gallon of gasoline. When chemists report \(\Delta\)E values for a reaction, they must therefore specify the amounts of chemicals that were used. There are two ways that chemists do this.
Option 1: Give the energy on a per mole (or per gram) basis, based on the most important chemical.
This option is used for physical processes that involve one chemical (such as evaporation or dissolving), and it is also commonly used for combustion reactions. For example, a chemist might say that \(\Delta E\) = -884.7 kJ/mol for the combustion of CH4(g). This statement tells us that if we allow one mole of CH4 to react with excess oxygen, we will get 884.7 kJ of energy. The negative sign of DE tells us that the chemical energy decreases by 884.7 kJ. This chemical energy is changed into thermal energy, so the chemicals create 884.7 kJ of thermal energy.
Option 2: Give a balanced chemical equation, and give the energy that is produced by the numbers of moles in the chemical equation.
This is the most common option. For instance, we might see the following statement:
\(\ce{2Ag^+(aq) + S^{2-}(aq) -> Ag2S(s)} \;\;\;\;\;\;\;\; \Delta E = -277 kJ\)
This statement tells us that when 2 moles of Ag+(aq) reacts with 1 mole of S2–(aq), we get 277 kJ of energy. We would not write “\(\Delta E\) = -277 kJ/mol for the reaction of Ag+ and S2–”, because the unit “kJ/mol” implies “kilojoules per one mole”, but we must use two moles of Ag+ to get our 277 kJ of energy.*
The ratio of energy to moles is a constant for any reaction. This fact allows us to calculate the energy change (and therefore the heat) when any amount of a chemical reacts. For instance, suppose that we react 0.25 mol of AgNO3(aq) with excess Na2S. We can set up the following proportionality:
\[\frac{-277 \text{ kJ}}{2 \text{ mol } \ce{AgNO3}}=\frac{\Delta E}{0.25 \text{ mol } \ce{AgNO3}}\nonumber\]
The values in the left-hand fraction come from the balanced equation above: we get 277 kJ of energy when we use 2 moles of AgNO3. The \(\Delta E\) in the right-hand fraction represents the energy we get when we use 0.25 moles of AgNO3. Solving this equation gives us \(\Delta E\) = -34.6 kJ, so we get 34.6 kJ of energy when 0.25 mol of AgNO3(aq) reacts with excess Na2S(aq).
PV work
The most practical way to measure \(\Delta E\) for a reaction is to carry out the reaction under conditions in which all of the energy is produced in the form of heat, because heat is much easier to measure than work. For most reactions, this is simple enough; most reactions will not do work unless the energy of the reaction is harnessed by some sort of machine. However, reactions that produce or consume gases are an exception. If the reaction produces a gas, the gas must push the atmosphere out of the way to make room for itself. This “pushing the atmosphere out of the way” involves motion (the reaction mixture expands) against a resistance (the atmospheric pressure), so it is work. The work that is done is given by the expression:
w = –Pexternal x \(\Delta\)V
where Pexternal is the pressure exerted by the surrounding atmosphere, and \(\Delta V\) is the change in the volume of the reaction mixture as it produces the gas. The negative sign is needed because the reaction mixture is doing the work; remember that when a system does work, its energy decreases. The figure below illustrates this type of work.
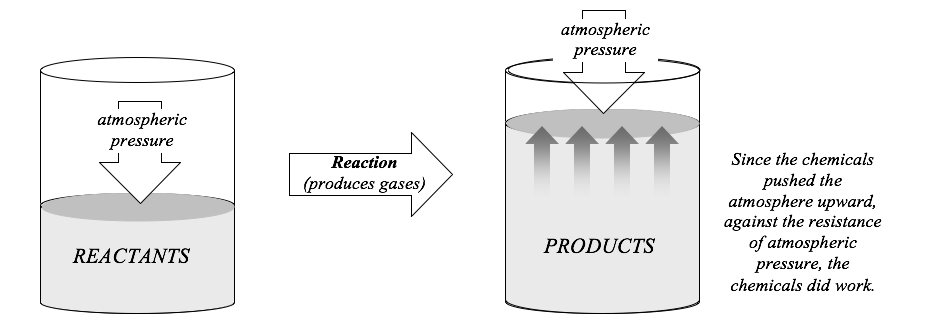
If a reaction consumes a gas, the reaction mixture becomes smaller as the gas is consumed, and the surrounding atmosphere must push inward to fill the empty space. In this case, the atmosphere does the work, but the equation
\w = –Pexternal x \(\Delta\)V
still applies. In this case, \(\Delta\)V is a negative number when the reaction mixture contracts, while w is positive, reflecting the fact that the surroundings are doing the work.
These types of work are called PV work, and they occur whenever we carry out a reaction that involves gas production or consumption in an open container. Therefore, if we want to ensure that no work is done during a reaction, we must carry out the reaction in a sealed container that cannot expand or contract. As long as the volume of the reaction mixture is constant, no PV work can be done. If the reaction produces a gas, the gas must squeeze itself into the existing space, but it cannot do work, because it cannot move the walls of the container. (Remember that work requires motion: if nothing moves, there is no work.) If the reaction consumes a gas, it will decrease the pressure in the container, but the surrounding atmosphere cannot collapse the walls of the container to equalize the pressures, so again, there is no work.
Whenever a reaction does PV work, the amount of heat that it produces or absorbs will be different from \(\Delta E\), because \(\Delta E\) equals the heat only when w = 0. For example, if we burn one mole of CH4 in an open container, the reaction produces 884.7 kJ of energy (as we saw above), but we get 889.7 kJ of heat from the reaction. At first glance, this seems impossible; how can the heat be larger than the overall energy? However, if we go back and look at the balanced equation…
CH4(g) + 2 O2(g) -> CO2(g) + 2 H2O(l)
…we see that this reaction converts three moles of gases (one mole of CH4 plus two moles of O2) into one mole of a gas (CO2) plus two moles of a liquid (H2O). Liquids have vastly smaller volumes than gases; at 25ºC and 1 atm, a mole of any gas occupies about 24.5 L under these conditions, whereas a mole of liquid water occupies just 0.018 L. Therefore, the total volume of the reaction mixture decreases, as shown below.
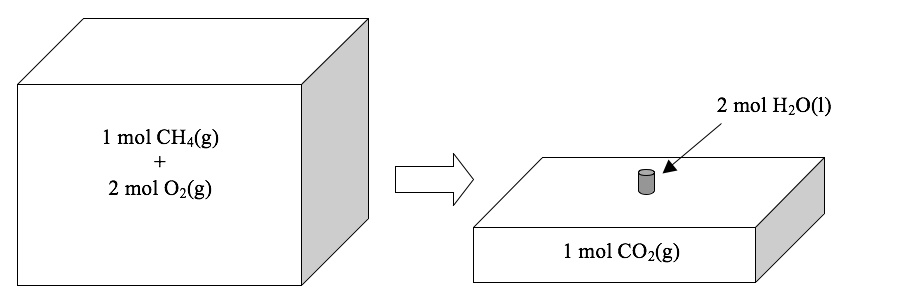
Note that the reaction mixture will not shrink on its own; the atmosphere must push inward and force the reaction mixture to become smaller. As the atmosphere compresses the reaction mixture, it does work. If the reaction occurred in a flexible container, we could see this work being done; for example, if the reaction took place in a balloon, the balloon would shrink dramatically. However, this work occurs whenever the external pressure is constant, regardless of whether we can see it.
In our example here, the atmosphere does 5.0 kJ of work. (You’ll see how to calculate this in a moment.) Since the surroundings are doing the work, w is a positive value from the standpoint of the reaction mixture; wreaction = +5.0 kJ. The First Law then governs the amount of heat we obtain:
\(\Delta\)Ereaction = qreaction + wreaction
-884.7 kJ = qreaction + 5.0 kJ
qreaction = -889.7 kJ
The physical meaning of these numbers is that the reaction converts 884.7 kJ of chemical energy into thermal energy, which comes out in the form of heat. The surrounding atmosphere contributes an additional 5.0 kJ as it compresses the reaction mixture. The reaction mixture converts this work into additional heat, so we get a total of 889.7 kJ of heat.
We can calculate the PV work using the equation wPV = –Pexternal x \(\Delta\)V. However, a much more convenient expression can be derived using the ideal gas law. To do so, we make a few assumptions:
- The internal pressure is essentially equal to the external pressure at all times, and the internal pressure is due entirely to the gases in the reaction mixture.
- The final temperature equals the initial temperature (which is reasonable if we want to extract all of the heat from a reaction).
- The volumes of any solids and liquids are so much smaller than the volumes of the gases that we can ignore them. We also make the substitution \(\Delta V\) = Vfinal – Vinitial.
Using these assumptions, we get:
wPV = –P\(\Delta V\)
= –(PVfinal – PVinitial)
= –(RTnfinal – RTninitial)
= –RT(nfinal – ninitial)
= –RTDngases
wPV = -RT∆ngases
By this derivation, we see that when the pressure remains constant, the PV work depends only on the temperature and the change in the number of moles of gases during the reaction. To get our value of wPV in joules, we must use R = 8.314 J/mol·K.
For example, here is how we would calculate the PV work for the combustion of one mole of CH4 at constant pressure. The balanced equation is:
CH4(g) + 2 O2(g) --> CO2(g) + 2 H2O(l)
The reaction converts 3 moles of gaseous reactants into 1 mole of gaseous product (plus some liquid water, which we ignore). Therefore, \(\Delta\)ngases = 1 mol – 3 mol = -2 mol. Using T = 298 K (25ºC), we get:
wPV = -(8.314 J/mol·K)(298 K)(-2 mol) = 4955 J = 4.955 kJ ≈ 5.0 kJ*
It is worth noting that PV work can only occur if a reaction produces more or fewer moles of gas than it consumes.** Many reactions do not involve gases at all, including precipitation reactions and acid-base reactions. A few reactions produce the same number of moles of gases that they consume, so the volume does not change. Here is an example:
C(s) + O2(g) --> CO2(g)
*If we know the values of P and V, we can also calculate the PV work by multiplying P x \(\Delta\)V, then using the conversion 1 L·atm = 101.325 J.
**Strictly, a tiny amount of PV work occurs even when a reaction involves only solids and liquids, but this PV work is so small that it does not produce a detectable change in the heat.
This reaction consumes a mole of gas (the O2) and produces a mole of gas (the CO2), so there is no change in the total number of moles of gases (\(\Delta\)ngases = 0) and therefore no PV work. This situation is uncommon, though; for most reactions that involve gases, the number of moles of gases changes during the reaction, so PV work can occur.
The amount of PV work that will occur during a reaction is typically much smaller than \(\Delta\)E for the reaction, so ignoring it doesn’t usually introduce a very large error. Here are a few examples of reactions involving gases, with their \(\Delta E\) and wPV values (calculated at room temperature, 298 K). Only for the last reaction is wPV a significant fraction of the overall energy change.
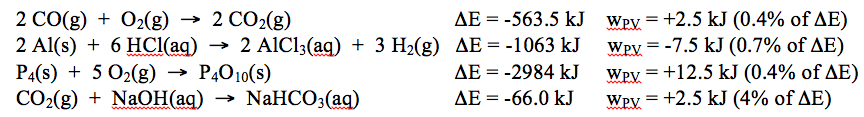
For most of these reactions, ignoring the PV work and assuming that all of the energy is transferred in the form of heat would not produce a significant error. However, chemists do not like to introduce errors into their work if they can avoid it. As we saw earlier, we can eliminate PV work by using a sealed, rigid container to carry out reactions that involve gases. Unfortunately, while carrying out reactions in sealed, rigid containers is feasible, it is not particularly practical for most applications. If we want to use a chemical reaction to heat our homes, or to power an engine, or to make a useful product, we need to expose the reaction mixture to the surroundings, and doing so allows PV work to occur. As a result, PV work presents an annoying complication whenever we want to calculate the heat that a reaction will give off or absorb.
Next, we will look at how chemists get around this problem…

