3.4.1.0: Molecular Shape (Problems)
- Page ID
- 210712
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)PROBLEM \(\PageIndex{1}\)
Explain why the HOH molecule is bent, whereas the HBeH molecule is linear.
- Answer
-
The placement of the two sets of unpaired electrons in water forces the bonds to assume a tetrahedral arrangement, and the resulting HOH molecule is bent. The HBeH molecule (in which Be has only two electrons to bond with the two electrons from the hydrogens) must have the electron pairs as far from one another as possible and is therefore linear.
PROBLEM \(\PageIndex{2}\)
Explain the difference between electron-pair geometry and molecular structure.
- Answer
-
Space must be provided for each pair of electrons whether they are in a bond or are present as lone pairs. Electron-pair geometry considers the placement of all electrons. Molecular structure considers only the bonding-pair geometry.
PROBLEM \(\PageIndex{3}\)
Why is the H–N–H angle in NH3 smaller than the H–C–H bond angle in CH4? Why is the H–N–H angle in \(\ce{NH4+}\) identical to the H–C–H bond angle in CH4?
- Answer
-
NH3 has a lone pair of electrons, which forces the bonds closer together, lessening the bond angle compared to species with 4 bonds and no lone pairs.
PROBLEM \(\PageIndex{4}\)
Predict the electron pair geometry and the molecular structure of each of the following molecules or ions:
a. BeH2
b. \(\ce{CH3+}\)
- Answer a
-
Both the electron geometry and the molecular structure are linear.
- Answer b
-
Both the electron geometry and the molecular structure are trigonal planar.
PROBLEM \(\PageIndex{5}\)
Identify the electron pair geometry and the molecular structure of each of the following molecules or ions:
a. CF4
b. BF3
c. BeCl2
- Answer a
-
Both the electron geometry and the molecular structure are tetrahedral.
- Answer b
-
Both the electron geometry and the molecular structure are trigonal planar.
- Answer c
-
Both the electron geometry and the molecular structure are linear.
PROBLEM \(\PageIndex{6}\)
What are the electron-pair geometry and the molecular structure of each of the following molecules or ions?
a. \(\ce{ClO2-}\)
b. PCl3
c. \(\ce{PH2-}\)
- Answer a
-
electron-pair geometry: tetrahedral
molecular structure: bent
- Answer b
-
electron-pair geometry: tetrahedral
molecular structure: trigonal pyramidal
- Answer c
-
electron-pair geometry: tetrahedral
molecular structure: bent (109°)
PROBLEM \(\PageIndex{7}\)
Identify the electron pair geometry and the molecular structure of each of the following molecules:
a. ClNO (N is the central atom)
b. CS2
c. Cl2CO (C is the central atom)
d. Cl2SO (S is the central atom)
e. SO2F2 (S is the central atom)
f. (g) \(\ce{ClOF2+}\) (Cl is the central atom)
- Answer a
-
electron-pair geometry: trigonal planar, molecular structure: bent (120°)
- Answer b
-
electron-pair geometry: linear, molecular structure: linear
- Answer c
-
electron-pair geometry: trigonal planar, molecular structure: trigonal planar
- Answer d
-
electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
- Answer e
-
electron-pair geometry: tetrahedral, molecular structure: tetrahedral
- Answer f
-
electron-pair geometry: tetrahedral, molecular structure: trigonal pyramidal
PROBLEM \(\PageIndex{8}\)
Draw the Lewis structures and predict the shape of each compound or ion:
a. CO2
b. \(\ce{NO2-}\)
c. SO3
d. \(\ce{SO3^2-}\)
- Answer a
-
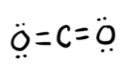
linear
- Answer b
-
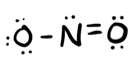
trigonal planar (bent 120)
- Answer c
-
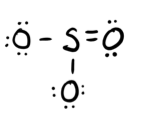
trigonal planar
- Answer d
-

tetrahedral (trigonal pyramidal)
- Click here to see a video of the solution
PROBLEM \(\PageIndex{9}\)
A molecule with the formula AB2, in which A and B represent different atoms, could have one of three different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion for each shape.
- Answer
-

PROBLEM \(\PageIndex{10}\)
A molecule with the formula AB3, in which A and B represent different atoms, could have one of two different shapes. Sketch and name the three different shapes that this molecule might have. Give an example of a molecule or ion that has each shape.
- Answer
-
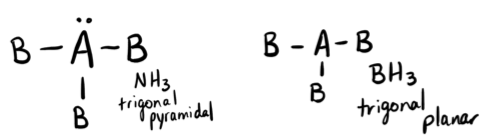
Have a video solution request?
Let your professors know here.
***Please know that you are helping future students - videos will be made in time for next term's class.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
Feedback
Think one of the answers above is wrong? Let us know here.

