Classification
Lewis acids and bases can be classified by designating them as hard or soft. Acids and bases are not strictly hard or soft, since many ions and compounds are classified as intermediate. The categorizations are based on three factors: (1) charge density, or charge-to-size ratio (2) polarizability (3) covalent vs. ionic nature of interactions.
Hard Acids/Bases:
In general, "Hard" acids and bases have a high charge density, are not very polarizable, and form bonding interactions that are more ionic in nature. These atoms and ions tend to have high charge and/or small radius.
- Typical Hard Acids: metal ions with high positive charges and smaller ionic sizes tend to be hard acids. Early transition metal ions in the 3d series tend to be hard Lewis acids.
- Typical Hard Bases: Small anions and neutral molecules; heteroatoms of the second row of the periodic table are typically hard (N,O,F). Some examples of hard acids and bases include: H+, O2-, OH-, F-, Fe3+, and Al3+. Oxygen atoms are always hard, and N atoms are usually hard.
Soft Acids/Bases:
In general, "Soft" acids or bases have a low charge density, are more polarizable, and form bonds that are more covalent in nature. These atoms/ions tend to have low charge and/or large radius.
- Typical Soft Acids: Transition metals with (+1) charge (such as Cu+) or that are in the late 4d and 5d series (like Cd2+ and Hg2+), are classified as soft. Soft acids often include transition metals in the second and third row of the periodic table that have a +1 or +2 charge, as well as late transition metals (especially those in the 4d and 5d series) with filled or almost completely filled d orbitals.
- Typical Soft Bases: Larger anions and neutral molecules. For example, I- and S2- are soft bases.
Intermediate Acids/Bases:
These are acids and bases with intermediate character, between hard and soft. For example, trimethylborane, Fe2+, and Pb2+ cations are intermediate acids, and pyridine and aniline are examples of intermediate bases.
An element can have different hard/soft character depending on its oxidation state and whether or not its polarizability is affected by surrounding atoms and bonds. The most extreme example is hydrogen, where H+ is a hard acid and H- is a soft base. Ni3+ (as in the layered compound NiOOH) is a hard acid, but Ni0 (as in Ni(CO)4) is a soft acid. Nitrogen is hard when it is an ammine (-NH2), but the nitrogen in pyridine is borderline due to increased polarizability imparted by the aromatic ring. The figures below show hard/soft trends for acids (left) and bases (right) in the periodic table. For bases, the major hard/soft discontinuity is between the 2nd row (N,O,F) and the rows below.
|
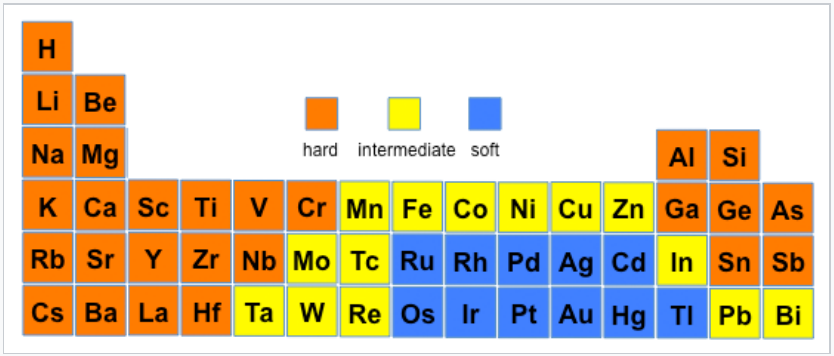
Hard-soft trends for acids
|
-
|
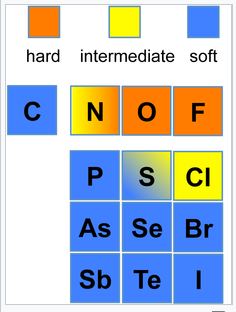
Hard-soft trends for bases
|
Like binds with Like
Hard acids interact more strongly with hard bases than they do with soft bases, and soft acids interact more strongly with soft bases than hard bases. Thus, the most stable complexes are those with hard-hard and soft-soft interactions. This tendency is illustrated in the table below, which shows the trend in formation constants for hard and soft acids. Hard acids bind halides in the order F- > Cl- > Br- > I-, whereas soft acids follow the opposite trend.
|
Log K1
|
fluoride
|
chloride
|
bromide
|
iodide
|
acid classification
|
|
Fe3+
|
6.0
|
1.4
|
0.5
|
-
|
Hard
|
|
Pb2+
|
1.3
|
0.9
|
1.1
|
1.3
|
Intermediate
|
|
Ag+
|
0.4
|
3.3
|
4.7
|
6.6
|
Soft
|
|
Hg2+
|
1.0
|
6.7
|
8.9
|
12.9
|
Soft
|
The softest metal ion in the periodic table is Au+(aq). It forms stable complexes with soft bases such as phosphines and CN-, but not with hard bases such as O2- or F-. The affinity of Au+ for the soft base CN- is high, and the resulting [Au(CN)2]- complex is so stable that gold (which is normally very difficult to oxidize) can be oxidized by oxygen in the air:
\[\ce{4Au_{(s)} + 8CN^{-}_{(aq)} + O2_{(g)} + 2H2O = 4[Au(CN)2]^{-}_{(aq)} + 4OH^{-}}\]
This reaction is used in gold mining to separate small flakes of Au from large volumes of sand and other oxides. Ag is similarly dissolved by air oxidation in cyanide solutions. The precious metals are then isolated from the solution using chemical reducing agents or by electroplating. The use of cyanide ion on a large scale in mining, however, creates a potentially serious environmental hazard. In 2000, a spill at Baia Mare, Romania resulted in the worst environmental disaster in Europe since Chernobyl. Cyanide, which is highly toxic, is gradually oxidized by air to the less toxic cyanate (OCN-) ion. On the laboratory scale, cyanide plating solutions are typically disposed of by using bleach to oxidize CN- to OCN-, and the metal is recovered as an insoluble chloride salt.
|

Netted solution pond next to cyanide heap leaching of gold ore near Elko, Nevada (1992).
|
The Au3+ ion, because of its higher positive charge, is a harder acid than Au+ and can form complexes with harder bases such as H2O and amines. In keeping with the "like binds like" principle, the compound AuI (soft-soft) is stable, but AuI3 (hard-soft) is unknown. Conversely, AuF has never been isolated but AuF3 (hard-hard) is stable.
Exercise \(\PageIndex{1}\)
Suggest which of the following ions is harder.
a) zinc(II) or mercury(II)
b) potassium(I) or copper(I)
c) iron(II) or iron(III)
- Answer a:
-
Zn(II), because it is smaller and less polarizable.
- Answer b:
-
K+, because it is less electronegative.
- Answer c:
-
Fe(III), because of the higher charge.
Exercise \(\PageIndex{2}\)
Suggest which of the following bases is softer.
a) Me3P or Me3N
b) chloride or iodide
c) amide (NH2-) or azide (N3-)
- Answer a:
-
Me3P, because phosphorus is larger and more polarizable than nitrogen.
- Answer b:
-
Iodide, which is larger and more polarizable than chloride.
- Answer c:
-
Azide, which has a more polarizable, delocalized pi bonding system.
There are some obvious HSAB applications in metallurgy and geology. Some common minerals of hard metals are rutile (titanium oxide, TiO2), dolomite (magnesium and calcium carbonate CaMg(CO3)2) and chromite (iron chromium oxide, FeCrO4). Fluoride, carbonates, oxides, phosphates and sulfates are examples of hard bases.
Some prevalent minerals of soft metals are galena (lead sulfide, PbS2) and cinnabar (mercury sulfide, HgS). Sulfides are the most common soft bases in geology, although the larger halides, like bromide and iodide, are also soft.
Some metals can pair with either hard or soft bases, particularly those metals from the middle of the transition metal group. For example, iron(III) is often found as hematite (iron oxide, Fe2O3), whereas iron(II) can also be found as pyrite (iron sulfide, FeS). Molybdenum(VI) can be found as powellite (calcium molybdenum oxide, CaMoO4), but the most commonly mined ore contains molybdenum(IV), found in molybdenite (MoS2).
Exercise \(\PageIndex{3}\)
Propose a formula for a plausible mineral containing each of the following ions.
a) zirconium(IV)
b) cadmium(II)
c) tungsten(VI)
d) zinc(II)
e) copper(I)
- Answer a:
-
ZrO2
- Answer b:
-
CdS
- Answer c:
-
WO3
- Answer d:
-
ZnS
- Answer e:
-
Cu2S
In biology, metals display aspects of hard & soft acid & base chemistry. Relatively hard potassium ions bind to oxygen atoms in DNA to help stabilize the helix structure. Calmodulin, which aids in calcium uptake, uses hard oxygen donors in aspartate and glutamate to bind to the Ca2+.
On the other hand, copper(I) is a soft acid. In poplar plastocyanin, which aids in transferring electrons during reactions in the plant cell, the copper ion is coordinated to two nitrogen-donating histidines and two sulfur donors, a cysteine and a methionine.
Many biologically important metal ions fall under the "borderline" category between hard and soft. Iron is one of the most abundant elements on earth, and many iron compounds play important roles in biology. Many biological compounds contain iron(II), which is able to bind well to both hard and soft ligands. Consequently, it is found with anionic oxygen carboxylate donors in methane monooxygenase, neutral and anionic nitrogen porphyrin donors in heme proteins, and sulfur cysteines and sulfides in ferridoxins and other iron-sulfur clusters.
Hard and soft acid and base phenomena have been studied using molecular orbital theory and other quantitative approaches. In MO theory, it has been shown that interactions between hard anions and cations are characterized by large HOMO-LUMO separations, whereas interactions between soft anions and cations are characterized by small HOMO-LUMO separations. In other words, hard acid-base interactions are dominated by more strongly ionic character, but soft acid-base interactions are dominated by more strongly covalent character.
Exercise \(\PageIndex{4}\)
Mercury ions, Hg(I) and Hg(II), are particularly poisonous. They can displace other metals from enzymes, so that the enzymes stop working.
a) are these ions hard or soft?
b) what amino acid residues would most likely bind to them?
- Answer a:
-
Hg(I) and Hg(II) are both large, polarizable ions. They are soft cations and should bind well to soft donors.
- Answer b:
-
The most common soft donor is a sulfur atom or sulfide ion; in amino acids, that suggests cysteine or methionine.
-
-
-
-
-
-
-
-
Exercise \(\PageIndex{5}\)
Enterobactin (below) is a molecule used by certain bacteria to bind iron(III) and transport it into the cell. The formation constant for the iron(III)-enterobactin complex is about 1049. Provide reasons why the formation constant is so high.
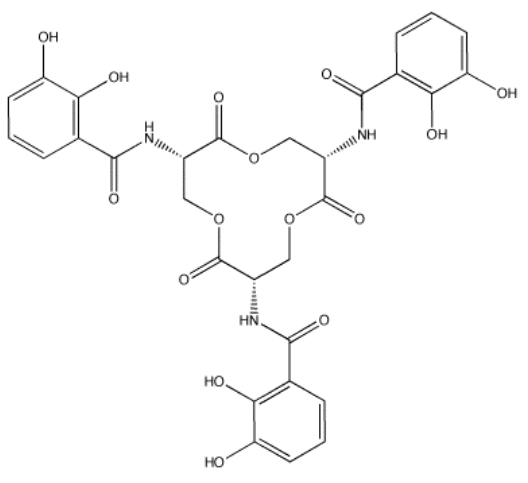
- Answer
-
Fe(III) is a hard cation and should bind well to oxygen donors. Enterobactin has several oxygen donors it could provide to the iron. In fact, there is a pair of OH groups on each of the benzene rings in enterobactin. These benzene rings with two OH groups next to each other are called "catechols". Because there are three of these groups in enterobactin, and there is enough space in between for the groups to fold around a central atom, enterobactin is a chelating (hexadentate) donor with a high binding constant.





