10.1: Proteins metabolism
- Page ID
- 234046
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Describe the metabolism of proteins.
- Know the importance of essential amino acids.
- Know the sources and function of common proteins in the diet.
Protein Metabolism
The main sources of amino acids for the human body are the proteins in our diet, the non-essential amino acids synthesized by the liver plus the amino acids that come from the own's body protein, which are being constantly degraded and resynthesized.
Protein digestion begins in the stomach (Figure \(\PageIndex{3}\)), where the action of gastric juice hydrolyzes about 10% of the peptide bonds. Gastric juice is a mixture of water (more than 99%), inorganic ions, hydrochloric acid, and various enzymes and other proteins. The pain of a gastric ulcer is at least partially due to irritation of the ulcerated tissue by acidic gastric juice.

Figure \(\PageIndex{1}\) The principal events and Sites of Protein Digestion
The hydrochloric acid (HCl) in gastric juice is secreted by glands in the stomach lining. The pH of freshly secreted gastric juice is about 1.0, but the contents of the stomach may raise the pH to between 1.5 and 2.5. HCl helps to denature food proteins; that is, it unfolds the protein molecules to expose their chains to more efficient enzyme action. The principal digestive component of gastric juice is pepsinogen, an inactive enzyme produced in cells located in the stomach wall. When food enters the stomach after a period of fasting, pepsinogen is converted to its active form—pepsin—in a series of steps initiated by the drop in pH. Pepsin catalyzes the hydrolysis of peptide linkages within protein molecules. It has a fairly broad specificity but acts preferentially on linkages involving the aromatic amino acids tryptophan, tyrosine, and phenylalanine, as well as methionine and leucine. Protein digestion is completed in the small intestine.
Amino Acids pool
Once the proteins in the diet have been hydrolyzed, the free amino acids join the non-essential amino acid synthesized in the liver and the amino acids recycled from the body's own proteins, constituting the amino acid pool now available for metabolic processes. Most of the amino acid pool is used for the synthesis of protein and other nitrogen-containing compounds such as DNA bases, neurotransmitters, hormones, etc. Under certain metabolic situations, amino acids can also be used as a source of energy by the body. It is worth mentioning that the human body cannot store amino acids. If the amino acids in the amino acid pool are not used for biological processes, they are degraded and the nitrogen excreted in the urine as urea.
Protein turnover
A balance between protein synthesis and protein degradation is required for good health and normal protein metabolism. Not all the amino acids needed for the biological function of the body need to be incorporated through the diet. When the proteins already present in the metabolism have complete their lifespan, they are also recycled. Protein turnover refers to the replacement of older proteins as they are broken down within the cell. Different types of proteins have very different turnover rates, depending on their particular function. Structural proteins such as college tend to have long half-life periods (in the range of years), while enzymatic protein have a shorter life span to adapt to the metabolic requirements of the body.
| Example protein half-lives | |
|---|---|
| Name | Half-Life |
| Collagen | 117 years |
| Eye lens crystallin | >70 years |
| Replication factor C subunit 1 | 9 hours |
|
40S ribosomal protein S8 |
3 hours |
| Ornithine decarboxylase | 11 minutes |
Once the protein have been hydrolyzed and amino acids recycled, these amino acids are added to the amino acid pool for further utilization.
Complete and Incomplete Proteins
Amino acids are classified into three groups namely: essential amino acids and nonessential amino acids
ESSENTIAL AMINO ACIDS
- Essential amino acids cannot be made by the body. As a result, they must come from food.
- The 9 essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
NONESSENTIAL AMINO ACIDS
Nonessential means that our bodies produce an amino acid, even if we do not get it from the food we eat. Nonessential amino acids include: alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Based on this classification of amino acids, proteins can also be classified as either complete or incomplete. Complete proteins provide adequate amounts of all nine essential amino acids. Animal proteins such as meat, fish, milk, and eggs are good examples of complete proteins. Incomplete proteins do not contain adequate amounts of one or more of the essential amino acids. For example, if a protein doesn't provide enough of the essential amino acid leucine it would be considered incomplete. Leucine would be referred to as the limiting amino acid, because there is not enough of it for the protein to be complete. Most plant foods are incomplete proteins, with a few exceptions such as soy. Table \(\PageIndex{1}\) shows the limiting amino acids in some plant foods.
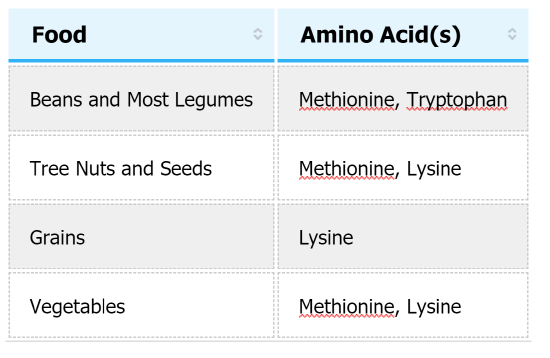
Table \(\PageIndex{1}\) Limiting Amino Acids in Some Common Plant Foods.
Complementary Proteins
Even though most plant foods do not contain complete proteins, it does not mean that they should be sworn off as protein sources. It is possible to pair foods containing incomplete proteins with different limiting amino acids to provide adequate amounts of the essential amino acids. These two proteins are called complementary proteins, because they supply the amino acid(s) missing in the other protein. A simple analogy would be that of a 4 piece puzzle. If one person has 2 pieces of a puzzle, and another person has 2 remaining pieces, neither of them have a complete puzzle. But when they are combined, the two individuals create a complete puzzle.

Figure \(\PageIndex{2}\) Complementary proteins are kind of like puzzle pieces.
Two examples of complementary proteins are shown below.

Figure \(\PageIndex{3}\) .Two complementary protein examples
It should be noted that complementary proteins do not need to be consumed at the same time or meal. It is currently recommended that essential amino acids be met on a daily basis, meaning that if a grain is consumed at one meal, a legume could be consumed at a later meal, and the proteins would still complement one another.
Summary
- Protein digestion begins in the stomach where hydrolysis of the protein linkages occurs with the action of gastric juices (mainly HCl ) and the active enzyme pepsin. Protein digestion is completed in the small intestine wherein other protein digesting enzymes are involved.
- Essential amino acids cannot be made by the body and must come from food.
- Complete proteins provide adequate amounts of all nine essential amino acids.
- Protein turnover refers to the replacement of amino acids in older proteins
- The amino acid pool is the total amount of amino acids from the diet, protein recycling, and non-essential amino acids produced by the body that is available for metabolic processing.
Source
Wikipedia
Protein turnover on Wikipedia. Retrieved sept. 28, 2020. Content adapted under the Creative Commons Attribution-ShareAlike License.
Contributors and Attributions
- Template:ContribLindshield
- Libretext: Basics of GOB Chemistry (Ball et al.)

