Thin Layer Chromatography
- Page ID
- 221871
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Thin layer chromatography (TLC) is a chromatographic technique used to separate the components of a mixture using a thin stationary phase supported by an inert backing. It may be performed on the analytical scale as a means of monitoring the progress of a reaction, or on the preparative scale to purify small amounts of a compound. TLC is an analytical tool widely used because of its simplicity, relative low cost, high sensitivity, and speed of separation.TLC functions on the same principle as all chromatography: a compound will have different affinities for the mobile and stationary phases, and this affects the speed at which it migrates. The goal of TLC is to obtain well defined, well separated spots.
Retention Factor
After a separation is complete, individual compounds appear as spots separated vertically. Each spot has a retention factor (Rf) which is equal to the distance migrated over the total distance covered by the solvent. The \( R_f\) formula is
\[ R_f= \dfrac{\text{distance traveled by sample}}{\text{distance traveled by solvent}} \]
The \( R_f\) value can be used to identify compounds due to their uniqueness to each compound. When comparing two different compounds under the same conditions, the compound with the larger \( R_f\) value is less polar because it does not stick to the stationary phase as long as the polar compound, which would have a lower \( R_f\) value.
\( R_f\) values and reproducibility can be affected by a number of different factors such as layer thickness, moisture on the TLC plate, vessel saturation, temperature, depth of mobile phase, nature of the TLC plate, sample size, and solvent parameters. These effects normally cause an increase in \( R_f\) values. However, in the case of layer thickness, the \( R_f\) value would decrease because the mobile phase moves slower up the plate.
If it is desired to express positions relative to the position of another substance, x, the \( R_x\) (relative retention value) can be calculated:
\[ R_x= \dfrac{\text{distance of compound from origin}}{\text{distance of compound x from origin}} \]
While \(R_f\) can never be greater than 1, \( R_x\) can be (i.e., faster than the reference compound \(x\).
Apparatus
Plates (Stationary Phase)
As stated earlier, TLC plates (also known as chromatoplates) can be prepared in the lab, but are most commonly purchased. Silica gel and alumina are among the most common stationary phases, but others are available as well. Many plates incorporate a compound which fluoresces under short-wave UV (254 nm). The backing of TLC plates is often composed of glass, aluminum, or plastic. Glass plates are chemically inert and best withstand reactive stains and heat, but are brittle and can be difficult to cut. Aluminum and plastic plates can be cut with scissors, but aluminum may not withstand strongly acidic or oxidizing stains, and plastic does not withstand the high heat required to develop many stains. Aluminum and plastic plates are also flexible, which may result in flaking of the stationary phase. Never under any circumstances touch the face of a TLC plate with your fingers as contamination from skin oils or residues on gloves can obscure results. Instead, always handle them by the edges, or with forceps.
The properties of your sample should be considered when selecting the stationary phase. As shown below in Table \(\PageIndex{1}\), silica gel can be exclusively used for amino acids and hydrocarbons. It is also important to note that silica gel is acidic. Therefore, silica gel offers poor separation of basic samples and can cause a deterioration of acid-labile molecules. This would be true for alumina plates in acidic solutions as well. It is important to note that there are differences between silica gel and alumina. Alumina is basic and it will not separate sample sizes as large as silica gel would at a given layer thickness. Also, alumina is more chemically reactive than silica gel and as a result, would require more care of compounds and compound classes. This care would avoid decomposition and rearrangement of the sample.
| Stationary Phase | Chromatographic Mechanism | Typical Application |
|---|---|---|
| Silica Gel | adsorption | steroids, amino acids, alcohols, hydrocarbons, lipids, aflaxtoxin, bile, acids, vitamins, alkaloids |
| Silica Gel RP | reversed phase | fatty acids, vitamins, steroids, hormones, carotenoids |
| Cellulose, kieselguhr | partition | carbohydrates, sugars, alcohols, amino acids, carboxylic acids, fatty acids |
| Aluminum oxide | adsorption | amines, alcohols, steroids, lipids, aflatoxins, bile acids, vitamins, alkaloids |
| PEI cellulose | ion exchange | nucleic acids, nucleotides, nucelosides, purines, pyrimidines |
| Magnesium silicate | adsorption | steroids, pesticides, lipids, alkaloids |
Chromatographic Columns is a good reference to learn more about the different types of columns and stationary phases.
Solvent (Mobile Phase)
Proper solvent selection is perhaps the most important aspect of TLC, and determining the best solvent may require a degree of trial and error. As with plate selection, keep in mind the chemical properties of the analytes. A common starting solvent is 1:1 hexane:ethyl acetate. Varying the ratio can have a pronounced effect of \(R_f\). \(R_f\) values range from 0 to 1 with 0 indicating that the solvent polarity is very low and 1 indicating that the solvent polarity is very high. When performing your experiment, you do not want your values to be 0 or 1 because your components that you are separating have different polarities. If the value is 0, you need to increase your solvent polarity because the sample is not moving and sticking to the stationary phase. If the value is 1, you need to decrease your solvent polarity because the compound was not able to separate.
If you know that one component of a mixture is insoluble in a given solvent, but another component is freely soluble in it, it often gives good separations. How fast the compounds travel up the plate depends on two things:
- If the compound is soluble in the solvent, it will travel further up the TLC plate
- How well the compound likes the stationary phase. If the compound likes the stationary phase, it will stick to it, which will cause it to not move very far on the chromatogram.
You should be able to determine which by looking at the \(R_f\) value.
Acids, bases, and strongly polar compounds often produce streaks rather than spots in neutral solvents. Streaks make it difficult to calculate an \(R_f\) and may occlude other spots. Adding a few percent of acetic or formic acid to the solvent can correct streaking with acids. Similarly for bases, adding a few percent triethylamine can improve results. For polar compounds adding a few percent methanol can also improve results.
The volatility of solvents should also be considered when chemical stains are to be used. Any solvent left on the plate may react with the stain and conceal spots. Many solvents can be removed by allowing them to sit on the bench for a few minutes, but very nonvolatile solvents may require time in a vacuum chamber. Volatile solvents should only be used once. If the mobile phase is used repeatedly, results will not be consistent or reproducible.
- A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate : butanol : acetic acid : water, 80:10:5:5.
- To separate strongly basic components, make a mixture of 10% NH4OH in methanol, and then make a 1 to 10% mixture of this in dichlormethane.
- Mixtures of 10% methanol or less in DCM can be useful for separating polar compounds.
Pipettes
- Spots are applied to the plate using very thin glass pipettes. The capillary should be thin enough to apply a neat spot, but not so thin as to prevent the uptake of an adequate quantity of analyte. Here is a popular method of producing TLC pipettes.
- Heat a glass capillary in the very tip of a Bunsen burner flame just until it becomes pliable and then pull the ends apart until the center of the capillary is significantly narrower. Snap this in half and use the thin end to apply spots.
Spotting and Developing
Developing a TLC plate requires a developing chamber or vessel. This can be as simple as a wide-mouth jar, but more specialized pieces of glassware to accommodate large plates are available. The chamber should contain enough solvent to just cover the bottom. It should also contain a piece of filter paper, or other absorbent material to saturate the atmosphere with solvent vapors. Finally, it should have a lid or other covering to minimize evaporation.
- Cut the plate to the correct size and using a pencil (never ever use a pen), gently draw a straight line across the plate approximately 1 cm from the bottom. Do not use excessive forces when writing on a TLC plate as this will remove the stationary phase. It is important to use a pencil rather than a pen because inks commonly travel up the plate with the solvent. An example of how black ink separates is shown in the section labeled "examples".
- Using TLC pipettes, apply spots of analyte to the line. Make sure enough sample is spotted on the plate. This can be done by using the short-wave UV. A purple spot should be seen. If the spot is not visible, more sample needs to be applied to the plate. If a standard of the target compound is available, it is good practice to produce a co-spot by spotting the standard onto a spot of the unknown mixture. This ensures the identity of the target compound.
- Place the plate into the chamber as evenly as possible and lean it against the side. Never allow the bulk solvent to rise above the line you drew. Allow capillary action to draw the solvent up the plate until it is approximately 1 cm from the end. Never allow the solvent to migrate all the way to the end of the plate.
- Remove the plate and immediately draw a pencil line across the solvent front.
- Use a short-wave UV light and circle the components shown with a pencil.
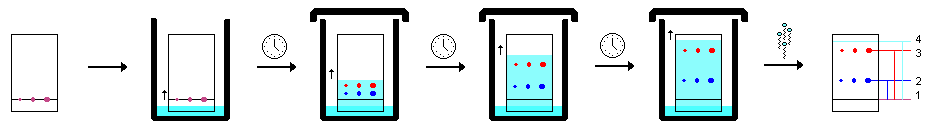
Visualizing
If fluorescent plates are used, a number of compounds can be seen by illuminating the plate with short-wave UV. Quenching causes dark spots on the surface of the plate. These dark patches should be circled with a pencil. For compounds which are not UV active, a number of chemical stains can be used. These can be very general, or they can be specific for a particular molecule or functional group.
Iodine is among the most common stains. Plates are placed in a jar containing iodine crystals, or covered in silica gel with iodine dispersed throughout, for approximately one minute. Most organic compounds will be temporarily stained brown. Some popular general use stains are Permanganate, ceric ammonium molybdate (CAM), and p-anisaldehyde. These can be kept in jars which plates are dipped into, or in spray bottles.
To develop a plate with permanganate, spray or dip the plate and heat it with a heat-gun. Hold the plate face up 10 to 20 cm above the heat gun until the bulk water evaporates. Then move the plate to 5 to 10 cm above the heat gun and heat it until white/yellow/brown spots appear. Overheating will turn the entire plate brown, obscuring the spots. If glass plates are used it is often easier to see spots through the backing because it is harder to overheat. CAM and p-anisaldehyde stained plates are developed similarly. Overheating CAM stained plates turns everything blue.
Common Problems in TLC
There are common problems in TLC that should be avoided. Normally, these problems can be solved or avoided if taught proper techniques.
- Over-large Spots: Spotting sizes of your sample should be not be larger than 1-2 mm in diameter. The component spots will never be larger than or smaller than your sample origin spot. If you have an over-large spot, this could cause overlapping of other component spots with similar \(R_f\) values on your TLC plate. If overlapping occurs, it would prove difficult to resolve the different components.
- Uneven Advance of Solvent Front: Uneven advance of the mobile phase is a common problem encountered in TLC. Consequences would be inaccurate Rf values due to the uneven advance of sample origin spots. This uneven advance can be caused by a few factors listed below.
- No flat bottom. When placing the TLC plate into the chamber, place the bottom of the plate on the edge of the chamber (normally glass container (e.g. beaker)) and lean the top of the plate along the other side of the chamber. Also, make sure that the TLC plate is placed in the chamber evenly. Do not tilt the plate or sit it at an angle.
- Not enough solvent. There should be enough solvent (depends on size of the chamber) to travel up the length of the TLC plate.
- Plate is not cut evenly. It is recommended that a ruler is used so that the plate is cut evenly.
Rarely, water is used as a solvent because it produces an uneven curve front which is mainly accounted for by its surface tension.
- Streaking: If the sample spot is too concentrated, the substance will travel up the stationary phase as a streak rather than a single separated spot. In other words, the solvent can not handle the concentrated sample and in result, moves as much of the substance as it can up the stationary phase. The substance that it can not move is left behind. This can be eliminated by diluting the sample solution. To ensure that you have enough solution, use a short-wave UV light to see if the spot is visible (normally purple in color), as stated earlier.
- Spotting: The sample should be above the solvent level. If the solvent level covers the sample, the sample spot will be washed off into the solvent before it travels up the TLC plate. An example is shown below.
Thin layer chromatography of three analgesics and caffeine under U.V. light was carried out in order to show the separation taking place. It is not a recommended technique in the laboratory. Due to the nature of the uv hazard polycarbonate safety spectacles (which absorb short wavelength U.V. light) and rubber gloves were worn throughout.
Five samples were run on a single TLC plate. The samples were (left to right on the plate):
- Ibuprofen (ICU)
- caffeine (CAF)
- u? = a commercial ‘pain relief’ medicine, used as an unknown
- Acetominophen (PAR)
- Aspirin (ASP)

The samples were dissolved in ethanol for spotting onto the plate. The TLC plate was run in an open beaker under short wavelength u.v. light using ethyl ethanoate as the eluting solvent.
.gif?revision=1)
The movement of the dark purple spots (samples) during the running of the plate can be observed in the animation. The original movie can be viewed here.

It is easy to see which are the two active ingredients in the unknown commercial pain relief medicine by comparison of the spots with the standard reference materials running on either side (caffeine and acetominophen).
TLC is very simple to use and inexpensive. Undergraduates can be taught this technique and apply its similar principles to other chromatographic techniques. There are little materials needed for TLC (chamber, watch glass, capillary, plate, solvent, pencil, and UV-light). Therefore, once the best solvent is found, it can be applied to other techniques such as High performance liquid chromatography. More than 1 compound can be separated on a TLC plate as long as the mobile phase is preferred for each compound. The solvents for the TLC plate can be changed easily and it is possible to use several different solvents depending on your desired results. As stated earlier, TLC can be used to ensure purity of a compound. It is very easy to check the purity using a UV-light. The identification of most compounds can be done simply by checking \( R_f\) literature values. You can modify the chromatography conditions easily to increase the optimization for resolution of a specific component.
TLC plates do not have long stationary phases. Therefore, the length of separation is limited compared to other chromatographic techniques. Also, the detection limit is a lot higher. If you would need a lower detection limit, one would have to use other chromatographic techniques. TLC operates as an open system, so factors such as humidity and temperature can be consequences to the results of your chromatogram.
References
- Touchstone, Joseph C. Practice of thin layer chromatography. 2nd ed. New York: Wiley, 1983.Print.
-
Geiss, Friedrich. Fundamentals of thin layer chromatography planar chromatography. Heidelberg: A. Hüthig, 1987. Print.
-
Touchstone, Joseph C. Practice of thin layer chromatography. 3rd ed. New York: Wiley, 1992. Print.
-
Figures: "Thin layer chromatography -." Wikipedia, the free encyclopedia. Web. 03 Dec. 2009. <http://en.Wikipedia.org/wiki/Thin_la...chromatography>.
Problems and Solutions
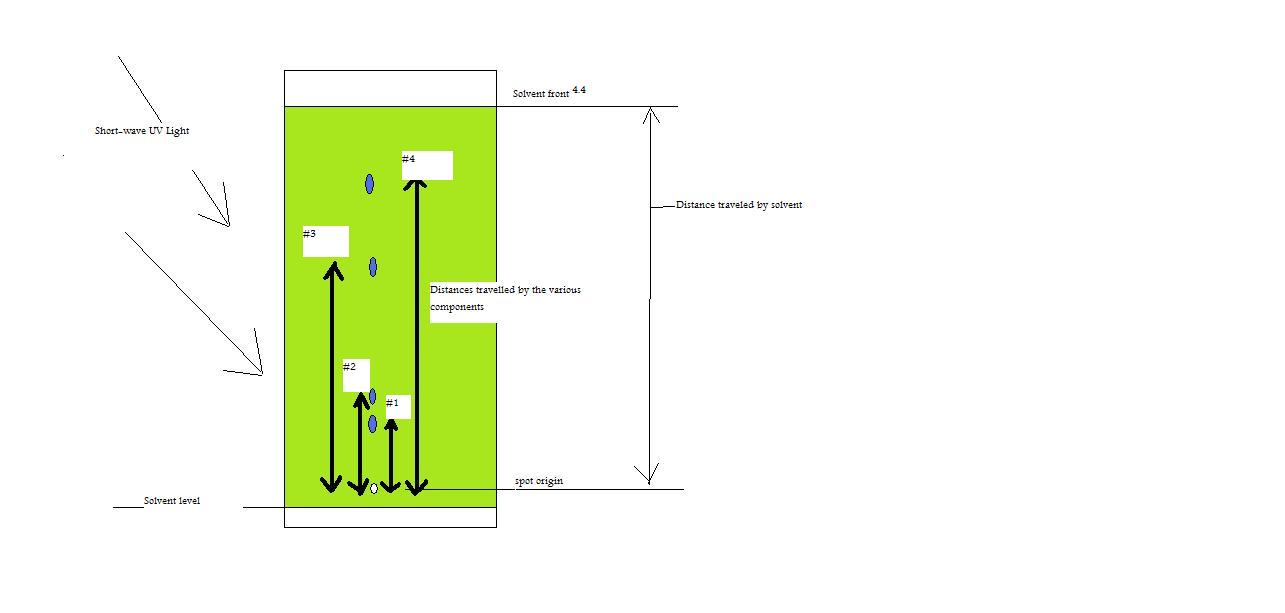
Figure 3: TLC plate under UV light with values for distance traveled of solvent and components.
Given:
#1=1.4 cm
#2= 1.5 cm
#3= 3.1 cm
#4= 3.6 cm
Using only the given information and the above figure, answer the problems listed below.
- What is the Rf value for component #2?
- What is the Rf value for component # 3?
- What is the relative retention value for components #1 and # 4, with # 4 being compound x?
- Using the answers from questions 1 and 2 and assuming that components 2 and 3 are different compounds, which component would be considered more polar? Explain.
Answers
- 1.5/4.4=0.34
- 3.1/4.4=0.70
- 1.4/3.6=0.39
- Component # 2 would be considered more polar because it has the lower Rf value, which means that it sticks to the stationary phase a lot stronger than component #3 and therefore moves slower in the mobile phase.