5.1: Autoionization, Neutralization, and an Introduction to Reaction Mechanisms
- Page ID
- 431551
This page is a draft and is under active development.
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
The chemical meaning of the term “pure” is simple: it is the condition of an element or compound when all of its component atoms or molecules are the same, the presence of isotopes notwithstanding. Thus if one speaks about pure water, the logical inference is that all of the particles in said water are H2O molecules. Surprisingly, water in such a state is never achievable in the real world; even if we could overcome the insurmountable practical difficulties of removing all trace contaminants from a given sample of water, it would still not be pure [1]. The reason? Molecules of water react with each other, albeit to a very small extent, to form ions [2]. In other words, pure water contaminates itself by spontaneously forming new species, and these reactions take place rapidly and constantly. Thus, even if we could get an absolutely pure sample of water, protected from air or any gases and held in an absolutely clean and inert container, ions would form almost instantly [3] because a constant tug-of-war is taking place between the oxygen atoms of water molecules for each other’s hydrogen atoms.
To understand this unexpected tendency of water to “self-contaminate”, we should first remind you that water molecules are polar, with the oxygen atoms bearing a partial negative charge and the hydrogen atoms being somewhat positive. There is therefore an attraction between the oxygen and hydrogen atoms of neighboring water molecules; such attractive forces between adjacent molecules are called intermolecular forces and are responsible for a host of phenomena of utmost importance in biochemistry, among other fields. As you would expect, water molecules tend to orient themselves to bring the oppositely charged atoms into as close proximity as possible as this minimizes their total potential energy. Chemical bonds are more like springs than rods, however, and a given hydrogen atom will oscillate between two oxygen atoms, albeit asymmetrically, staying closer to the one it is covalently bonded to. Occasionally, though, a hydrogen atom will shift position, actually breaking the covalent bond that existed initially and forming a new covalent bond with what was the more distant oxygen atom. The reaction is described by the balanced equation below and is illustrated in Figure 5-2.
\[ \ce{2 H2O <=> H3O+ + OH- }\]

Figure 5-2. The autoionization of water is a simple “proton transfer” from one neutral water molecule to another, resulting in the formation of the hydronium cation, H3O+, and the hydroxide anion, OH-. The double arrows indicate that each step is reversible.
Note that when the hydrogen atom departs from the oxygen to which it is bonded initially, it does so without either of the electrons that composed the covalent bond. Read that last sentence again, because it is of critical importance. A hydrogen atom without its electron is the hydrogen cation, H+, a unique cation in that it has absolutely no electrons. It is simply the nucleus of the hydrogen atom and, because the nuclei of hydrogen atoms contain just one proton, H+ is commonly referred to simply as a “proton”. The reaction described above is therefore known as a proton transfer and is among the most important classes of chemical reactions. Simple bookkeeping reveals that such a process must result in the formation of products that bear nonzero charges; when a positive ion is removed from a neutral molecule, what remains must be negative. Thus the water molecule that loses the proton will end up with a negative charge and has the formula OH-. Note the formal -1 charge on the oxygen. This is the hydroxide ion and is an example of a polyatomic ion, as distinct from the monatomic ions such as chloride, Cl-. Similarly, the water molecule that accepts a proton will become a cation, the hydronium ion, H3O+, which has a formal +1 charge on the oxygen. Because this reaction entails the generation of ions from neutral molecules of a particular compound it is called an autoionization reaction; water is not the only compound capable of such behavior but it is by far the most important [4]. It forms the basis of all aqueous acid-base chemistry, the implications and applications of which are impossible to overstate. We return to the idea shortly, but first want to introduce a widely used formalism to describe the pathway of chemical reactions, one that we use throughout the remainder of this text.
The process of proton transfer depicted in Figure 5-2 is an example of a reaction mechanism, which is a sequence of events by which a chemical reaction takes place. Reaction mechanisms may consists of a single step, as is the case with autoionization, or may consist of many distinct steps. No matter how complex a material transformation may be, it can be broken down to a series of elementary steps, reactions that involve the breakage and or formation of a chemical bond, or the simultaneous breakage and formation of two or more bonds. The autoionization reaction involves the simultaneous breakage of one O-H bond and the formation of a new one. Because bonds typically consist of shared electron pairs, elementary steps are often drawn using what is called curved arrow notation to focus our attention on the electrons of the bonds undergoing changes. An example of this notation showing the bond breakage and formation of autoionization is shown in Figure 5-3. Here, the red arrow indicates what is referred to as an “attack” of a lone pair of electrons on a hydrogen atom of a different water molecule. You interpret this in the following way: those electrons at the tail end of a given arrow will form a new covalent bond with the “target” atom at the head of the same arrow, in this case the hydrogen atom of a neighboring water molecule. Because hydrogen can only accommodate two electrons, it cannot form a new bond without breaking the one it was initially part of, so the blue arrow in Figure 4-3 shows that the electrons that composed the original O-H covalent bond retreat to the oxygen, thereby ending up as a third lone pair of electrons on that atom.
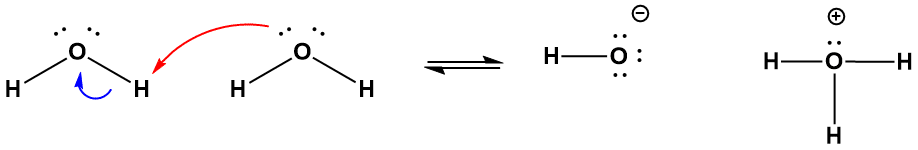
Figure 5-3. The arrow notation depiction of water’s autoionization reaction; note that the arrows do not indicate any motion of the atoms or molecules, but how electrons are directed in the formation or cleavage of covalent bonds.
It is worth emphasizing that the arrows employed in arrow notation do not imply any literal movement of particular atoms, a mistake commonly made by those just getting acquainted with the formalism. Rather, they are meant to explicitly show which atoms will be bonded covalently, or where a new lone pair of electrons will reside, when that particular elementary step is completed. For a bit more practice in interpreting this notation, look at Figure 5-4 and prove to yourself that it shows the reverse reaction of that shown above, that is, it shows a neutralization reaction, the formation of two neutral water molecules from hydroxide and hydronium ions.

Figure 5-4. The curved arrow notation for the reaction between hydroxide and hydronium.
Arrow notation is commonly used to illustrate individual steps of reaction mechanisms. Mechanisms can be speculative because it is very difficult, as an experimental matter, to obtain direct evidence for steps that may take place over a time scale that is far shorter than microseconds. Nevertheless, evidence can be collected to support on plausible pathway over an alternative one and, over time, a fairly clear picture can emerge concerning what steps are involved ina given reaction pathway. Understanding reaction mechanism can be incredibly useful when trying to manipulate matter effectively and predict reaction outcomes. We will return to this idea many times in coming chapters. Currently, our aim is simply to introduce the idea of how atoms or ions can “change hands” during a reaction and the arrow notation is an useful way to help you visualize such processes.
Footnotes and References.
[1] All gases, for example, are at least somewhat soluble in water, and water will inevitably dissolve small amounts of material and surface contaminants from any vessel in which it is placed.
[2] How small? For about every 500,000,000 water molecules in “pure” water, there is only one pair of ions formed.
[3] “Almost instantly” does not mean instantaneously; all chemical reactions are physical events that take a finite period of time. The reaction we are referring to presently, however, is quite rapid and will proceed in much less than a second. The study of chemical reaction rates, called chemical kinetics, is an important field of study in chemistry.
[4] See Problem 5-X at the end of this chapter for a couple of other examples.

