5.2: The pH Scale
- Page ID
- 431622
As stated earlier, there are many chemical and physical systems that lend themselves to a logical and systematic treatment of equilibrium, an approach we outline later. For the moment, however, we need to define some terms, a few of which may be familiar to you. Let’s go back to the autoionization of water for a moment. Recall that in water that is impossibly pure, where there is no hydronium or hydroxide present, autoionization proceeds faster than the opposing neutralization until the concentrations of hydronium and hydroxide reach 1.0 × 10-7 M; at that point the concentrations of these ions reaches a point where the reverse reaction is fast enough to balance the autoionization process and a steady state condition is achieved. But the relationship between the concentrations of these ions is more complex than the above may imply.
Recapping a key idea from the previous section: water spontaneously reacts to form a relatively low concentration hydroxide and hydronium ions in the reversible process described by the following chemical equation:
\[ \ce{2 H2O <=> H3O+ + OH- } \nonumber \]
If we performed a thought experiment where we start with "impossibly pure water", meaning pure water that has no hydronium or hydroxide, and allow the autoionization to commence, these two ions will be generated in equal amounts in accord with the above balanced equation until they reach 1.0 × 10-7 M. At this point the system reaches steady-state, a condition wherein the rates of the opposing processes are equal and no further change in their concentration is observed. Note that these concentrations refer to those in pure water, that is, water into which no other compounds have been introduced. Acids and bases are compounds that, when dissolved, change hydronium and hydroxide concentrations. Specifically, acids increase the concentration of hydronium ions, while bases increase the concentration of hydroxide ions [7]. Interestingly, the concentrations of these two ions do not vary independently; if one of them increases, the other must decrease. Thus, solutions that have relatively high concentrations of hydronium must have relatively low hydroxide concentrations and vice versa. Solutions in which [H3O+] > [OH-] are acidic, while those where [OH-] > [H3O+] are basic. When [H3O+] = [OH-], as in the case of otherwise pure water and solutions of compounds that have no acidic or basic properties, the solutions are neutral.
Perhaps surprisingly, there is an inverse relationship between the hydronium and hydroxide concentrations in all aqueous solutions that can be written as,
\[ [\ce{H3O+}] [\ce{OH-}] = 1.0\ ×\ 10^{-14}\ M^2 \]
Because of the above relationship, if you know of the two concentrations of hydronium or hydroxide, you can calculate the other. We explain how acids and bases change the concentrations of these ions below, but pause here to offer a few examples of how the above equation is used and to introduce the pH scale.
Problem 5-1. The hydronium ion concentration of a given solution is 9.2×10-9 M. Calculate the hydroxide ion concentration of this solution.
Solution
Rearrange equation 5.2.1 to derive the equation for [OH-], as follows:
\[ [\ce{OH-}]\ =\ \dfrac{1.0×10^{-14}M^2}{[\ce{H3O+}]} = \ \dfrac{1.0×10^{-14}M^2}{9.2×10^{-9} M} = 1.1×10^{-6} M \nonumber \]
Problem 5-2. Calculate the hydroxide concentrations of solutions in which:
a) [H3O+] = 2.5×10-5 M
b) [H3O+] = 1.1×10-13 M
c) [H3O+] = 4.8×10-1 M
Problem 5-3. Calculate the hydronium concentrations of solutions in which:
a) [OH-] = 7.5×10-6 M
b) [OH-] = 2.5×10-3 M
c) [OH-] = 7.0×10-7 M
Problem 5-4. Of the above six solutions, which are acidic? basic? Are any of them neutral?
From the above examples, you can see that the range of hydronium ion concentrations that are possible span many orders of magnitude. As a matter of fact, different solutions that are commonly encountered in everyday contexts can have hydronium ion concentrations that differ by about 14 orders of magnitude: the hydronium ion concentrations of the most acidic solutions you might use (such as in certain cleaning solutions or automobile batteries) is more that a trillion times that of the most basic solutions you may encounter (such as in oven cleaners). Because the concentration of hydronium and hydroxide ions in an aqueous solution plays such an important role in determining its chemical properties (which is why it is measured routinely in contexts such as diverse as medical tests and swimming pool maintenance!) a way of expressing the acidity or basicity of solutions that does not require scientific notation – the pH scale – is widely used. Most people are somewhat familiar with the pH scale (it is frequently used in advertising, such as in the promotion of "pH balanced shampoos"). pH stands for "power of hydronium" and is expresses the hydronium ion concentration of a solution according to the following equation:
\[ \text{pH} = -log[\ce{H3O+}] \]
Given the above equation, the pH scale eliminates the need for scientific notation because it is a logarithmic scale: each pH unit corresponds to a factor of ten in hydronium concentration. Thus a solution that has a pH of 3 has ten times the hydronium ion concentrations as a solution with a pH of 4, one hundred times that of a solution with a pH of 5, and 10,000 times that of a solution that has a pH of 7. You may have heard that a pH of 7 indicates neutrality. Why is this? It is not the mid-point of a randomly chosen scale, but a direct application of equation 5.3.2. Recall that pure water has [H3O+] = [OH-] = 1x10-7 M; using this concentration in the pH equation yields a value of 7. In addition, there is a common misconception that the pH scale goes from 0 to 14. There are, in fact, no rigidly defined endpoints to the scale. Negative pH values are possible, as are values above 14. There is nothing magic about the number 0 on the pH scale that makes correspond to a maximum concentration of hydronium, as the following examples show; the concentrations in these examples are easily achievable in the lab.
Problem 5-5. Calculate the pH of solutions that have the following concentrations of hydronium: a) 1.0 M; b) 2.0 M; c) 5.0 M.
Before explaining why acids increase the concentration of hydronium ions and why bases increase the concentrations of hydroxide, we need to define a few terms to avoid confusion. The above description of acids and bases is actually just one of several definitions of these terms. There are actually three distinct sets of definitions of acids and bases that are commonly employed (and several other more niche definitions). The above is the so-called Arrehnius definition. It is a special case of a more general definition called the Brønsted-Lowry definition that states:
- acid: a compound that acts as proton (H+) donor in a chemical reaction
- base: a compound that acts as a proton acceptor (H+) in a chemical reaction
For most reactions that occur in water, the Brønsted-Lowry definition is probably the most useful and the one we will primarily employ. The Arrhenius definition is more specific in that it specifies the effect of a compound on the hydronium and hydroxide concentration in water. But the Brønsted-Lowry definition allows for other types of proton-transfer reactions that don't necessarily involve water. For example, some proton-transfer reactions can take place between gaseous molecules, and others can take place in solvents other than water and which do not involve hydronium or hydroxide. The Brønsted-Lowry definition, in turn, is a more specific case of the Lewis acid/base definition, that focuses on electron pair donors and acceptors. We we will explain this theory later in this text as it is not necessary to understand the points made below.
To illustrate how the above definitions apply to some simple acid/base reactions we'll use the case of a "generic acid", HA, meaning a molecule that serves as a proton donor to a base (equation 5.3.2). In this reaction water serves as the base because it accepts the H+ from the acid how an acid increases the hydronium ion concentration, consider the proton transfer process described by equation 5.3.2 and illustrated by Figure 5-6. Specifically, acids increase the H3O+ concentration of water by donating an H+ ion to water, or protonating it. In the figure, the compound written as “HA” in the balanced equation represents a generic acid, that is, any compound capable of donating a hydrogen ion to water. The electrons of one of the nonbonding pairs on oxygen of a water molecule serve to abstract the hydrogen ion from the acid, yielding hydronium, and A-, which is all that remains of HA after the positive hydrogen ion is removed.
\[ \ce{HA (aq) + H2O (l) <=> H3O+(aq) + A- (aq)} \]
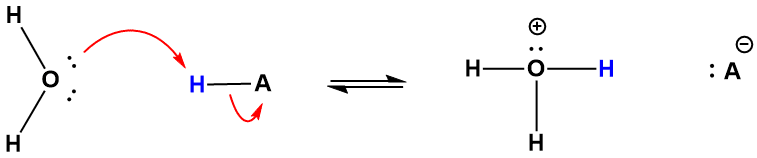
Figure 5-6. The proton transfer from a generic acid, HA, to water; water serves as the base in this reaction. The products are the hydronium ion, which is the conjugate acid, and A-, the conjugate base. The reverse reaction also takes place and such systems typically reach equilibrium very quickly.
Figure 5-7. Several common carboxylic acids (systematic names are in parentheses); note the COOH functional group present in all of these compounds. The hydrogen of the COOH group is by far the most acidic hydrogen in each of these structures and will be preferentially removed by bases.
There are thousands of compounds that act as acids, many of them occurring naturally. Accordingly, HA is useful shorthand notation for describing all of these compounds. Note that acids are defined by what they do more than what they are, thus anything that acts as HA does in Figure 5-6, that is, as a proton donor, is by definition, an acid. We introduced an entire family of such compounds back in Chapter 1: the carboxylic acids. Recall that these have the general formula, RCOOH; linoleic acid, described at length back then, as were a few of the simpler members of this class of compound. Structures of several of the more commonly encountered carboxylic acids are shown in Figure 5-7. Amino acids, the molecular building blocks of proteins, also have carboxylic acid functional groups. Acetic acid is probably the most familiar organic acid to people. It is the clear, colorless compound that gives vinegar its distinctive and pungent aroma. It reacts with water, albeit sparingly, donating an H+ ion to form the hydronium ion as shown in Figure 5-8. This is an equilibrium process that reaches steady state when only about 0.4% of the acetic acid molecules are deprotonated [8].
Figure 5-8. The reaction of water with acetic acid to form hydronium and the acetate ion; in the reverse direction acetate serves as the base and hydronium the acid. The reaction reaches equilibrium when about 0.4% of the acetic acid is converted to acetate; the extent of the reaction depends, however, on the concentration of acetic acid, and the 0.4% figure is valid when the acid is present at 1 M.
What about bases? How do they increase the concentration of hydroxide ions in water? The process is similar to that shown in Figure 5-6 but the role of water is reversed. Rather than serving as the acceptor of the proton from an acid, it serves and the donor of a proton, as equation 5.3.3 indicates. Here, the generic base, B, serves as the proton acceptor, forming the protonated form, BH+. Water serves as the acid and forms the hydroxide ion by losing an H+ ion. Figure 5-9 illustrates the reaction of a specific base, ammonia, NH3; the ammonium ion, NH4+ is formed along with hydroxide when ammonia accepts a proton from water. This reaction reaches equilibrium when about 0.4% of the dissolved ammonia is converted to ammonium.
\[\ce{B (aq) + H2O (l) <=> BH+ (aq) + OH- (aq)} \]
Figure 5-9. The reaction between water and ammonia, NH3 , to form hydroxide and ammonium, NH4+, ions. In the reverse direction ammonium serves as the acid and protonates hydroxide to make water.
We can describe the neutralization of acids and bases using the terms described above. Hydronium ions, H3O+, which are present in excess in acidic solutions, serve as the acid because they donate H+ ions to hydroxide, OH-, the prevalent species in basic solutions. Describing the autoionization reaction is a bit trickier: one water molecule serves as the acid, while the other serves as the base. This is an example of an important point: stating that any particular species is an acid or base says something explicit about what it does rather than what it is. Thus one water molecule serves as the acid and another water molecule serves as the base in autoionization. Water is not unique in its ability to act as an acid and a base, and such compounds are referred to as amphoteric; these types of species play an important biological role as they buffer blood, plasma, and cellular fluids against large changes in acidity.
To end this section we introduce a few new terms that are frequently used when discussing acid/base reactions. As we've stated several times, many of these reactions proceed in both directions. The reaction between acetic acid and water forms hydronium and acetate, while acetate and hydronium react to form water and acetic acid (Figure 5-10).
Footnotes and References.
[6] These are the equilibrium concentrations at 25°C; the extent of autoioniziation increases with temperature, so the equilibrium concentrations of these ions will be greater at higher temperatures.
[7] As we will see, this is only one way to define acids and bases; it is referred to as the Arrhenius definition and is a specific case of the Brønsted-Lowry definition. The latter defines an acid as any species that donates a proton to another species, the base; note that there is no necessity that water be either the proton donor or acceptor as is the case with the Lowry definition. Finally, the Lewis definition of acids and bases is by far the most general; it defines an acid as a species that “accepts” a pair of electrons in the formation of a new covalent bond, and the base is the electron pair donor. Thus, all bases must have a lone pair of electrons and all acids must be able to accommodate two electrons to form a bond. You can see that this is consistent with the Brønsted -Lowry definition in that H+, the acid, can accommodate two electrons to form a bond. The Lewis definition is not widely used in aqueous systems but is very helpful when considering many organic reactions that take place in nonpolar media.
[8] This value is valid for solutions that are ~5% acetic acid by weight, fairly typical values for commercially available vinegar; the actual extent of deprotonation depends on temperature and concentration.

