4.5: Electron Dot Structures of Organic Compounds
- Page ID
- 417801
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)So far we have focused primarily on two simple types of molecular compounds: homodiatomic molecules such as H2, F2, N2 and O2 [22], and binary compounds such as water. But we saw in the previous section that hydrogen and oxygen can also form another compound, namely hydrogen peroxide, H2O2. Both H2O and H2O2 are binary compounds of these elements, but the ratio of the elements is different in them: the H:O ratio in water is 2:1, but 1:1 in H2O2. How many other binary compounds between these two elements exist? Based on the bonding principles we’ve presented thus far, one could reasonably speculate that extended chains of oxygen could exist, in much the same way carbon atoms can form long chains as we saw with alkanes in Chapter 1. For example, the molecule H2O5 (Figure 4-22) seems like a reasonable candidate for a stable molecule in that the octets of all of the oxygen are satisfied (and all the oxygen atoms have 2 bonds, as expected because the valence of oxygen is two) and each hydrogen atom has its preferred dyad (and meets its normal valence of one). But this particular structure has never been observed and therefore serves as an important example: just because you can draw what seems to be a good electron dot structure for a molecule does not mean it will exist. A good electron dot structure, one that satisfies all atoms’ octets, you might say is a necessary but not sufficient condition in predicting molecular stability. The instability of H2O5 can be attributed to all of those non-bonding lone pairs on adjacent oxygen atoms; lone pairs of electrons repel each other on account of their like charges, and when there are too many of them adjacent to each other they tend to destabilize whatever structure they are part of. Hydrogen peroxide is similarly destabilized and it is much more reactive than water because of it; the effect is not so severe that it prevents H2O2 from existing, however. But chains of oxygen longer than three atoms have never been observed. Thus the number of binary compounds for hydrogen and oxygen, indeed for most pairs of elements, is quite limited. Not so for carbon, however.
Figure 4-22: Two structures of the hypothetical (and non-existent) molecule H2O5; (left) an electron dot structure that explicitly shows all bonding and non-bonding electron pairs; (right) a simplified structural formula of the same compound; such extended chains of oxygen atoms are not stable due to the extensive electrostatic repulsion of so many lone pairs of electrons.
What makes carbon so special? It can form, literally, an infinite number of binary compounds with hydrogen. To see why, look at its electron dot structure (Figure 4-16 b); it has four electrons and therefore needs to form four covalent bonds to complete its octet and doing so it will not leave any destabilizing lone pairs. It can do so by making bonds solely with hydrogen, to yield methane, CH4. But it can also make single bonds to other carbon atoms without introducing any lone pairs either. Carbon, therefore, has many viable structures by which it can form bonds to hydrogen and other carbon atoms to satisfy its octet without incurring unduly high energetic costs.
Consider the following: you are tasked with drawing an electron dot structure of an alkane having the formula C4H10. What is a systematic approach? You know that hydrogen can only form one bond so, therefore, hydrogen atoms cannot be incorporated into the molecular "backbone". Begin, therefore, with the four carbon atoms and join them with single bonds (Figure 4-23, left). This leaves a total of ten unpaired electrons along the length of the chain where the hydrogen atoms must go. You would follow the same approach for drawing Lewis structures for any alkane. The only wrinkle would arise from the possibility of isomers. Because carbon can make numerous bonds to other carbon atoms, ranging from zero to four, the possibility of branching exists. In the above example, we could have made a backbone of three carbon atoms, and placed the fourth carbon off the center of the backbone (Figure 4-23, right); doing so would still leave ten available electrons to bond to hydrogen and it would give a structure in which all of the carbon atoms have complete octets. Both structures have the formula C4H10 and both represent stable compounds. You therefore need more than the simple molecular formula to unambiguously represent all but the simplest of alkanes, indeed of many organic structures.
| Figure 4-23. Stepwise approaches to drawing Lewis structure of isomeric alkanes butane (left) and 2-methylpropane (right). In each case the carbon atoms comprising the backbone are drawn first, then the terminal hydrogen atoms are placed on the periphery; note that all carbon atoms have complete octets in the final structures. |
Here’s another problem: draw a Lewis structure for ethene, C2H4. Using the approach outlined above, you would have two carbon atoms joined forming the tiniest of backbones. Doing so leaves six unpaired electrons where you could place hydrogen atoms; but the formula calls for only four hydrogen atoms which, when distributed equally between the carbons, leaves each carbon with seven electrons and a lone electron (Fig 4-24). We encountered something similar when drawing the electron dot structure of O2 (Figure 4-21). Specifically, after making the first bond, we were left with a remaining lone electron on both atoms. Just as the oxygen atoms formed a double bond to satisfy their octets, so will the carbon atoms in C2H4. It's worth noting that C2H4 is isoelectronic with O2 (they both have a total of 12 valence electrons) and species that isoelectronic with each other will often show similar bonding patterns because there are only a limited number of ways a given number of electrons can be distributed while still obeying the octet rule. Note that we have already seen several isoelectronic species such as fluorine and the hydroxyl radical, both of which are also isoelectronic with the methyl radical, CH3.
Figure 4-24: Building the Lewis structure for C2H4; there are not enough hydrogen atoms to satisfy the octets for singly bonded carbon atoms, so the latter satisfy their valence of four by forming a double bond with the remaining lone electrons.
Problem 4-13. In the above paragraph, it was implied that the four hydrogen atoms of C2H4 should be distributed equally between the two carbon atoms. Can they also be distributed unequally between the carbon atoms and make a stable compound? Why or why not?
We can use the idea of isoelectronic structures to introduce a new functional group as follows. Just as there is a hydrocarbon that is isoelectronic with O2, there is also one that is isoelectronic with N2: what is it? Answer: each nitrogen atom has five valence electrons so, to be isoelectronic with N2, we need two hydrocarbon fragments that also have five valence electrons each. The only possibility is CH, which has 4 electrons from carbon and one from hydrogen. Figure 4-25, top, illustrates this by showing the electron dot structure for a single nitrogen atom along with that for a CH fragment. Just as nitrogen satisfies its octet by forming a triple bond, so will the CH fragment, forming a carbon-carbon triple bond (Figure 4-25, bottom). This compound, with the formula C2H2, is called ethyne and is an example of an alkyne, the term given to compounds featuring carbon-carbon triple bonds. Alkynes share many chemical properties with alkenes because they are both unsaturated hydrocarbons.
|
|
Figure 4-25: (top) the CH fragment is isoelectronic with a single nitrogen atom, as both have five valence electrons, albeit those of the CH fragment come from two atoms, not one. (bottom) Like individual nitrogen atoms, CH fragments can each satisfy the octets of their carbon atoms by forming triple bonds (see Figure 4-21 for comparison). |
Other organic structures can be represented using the same concepts discussed above. We’ll look at some of the compounds we introduced in Chapter 1 to illustrate, starting with alcohols. We’ve already seen the hydroxyl radical: it has seven valence electrons and needs to form one covalent bond to satisfy oxygen’s octet. To draw butan-1-ol, we simply need to remove a hydrogen atom from butane, and replace it with a hydroxyl group (Figure 4-26); this gives the carbon and the oxygen bonded to it each eight valence electrons. Note that in the structure, all of the carbon atoms have a valence of four and that of oxygen is two.
|
Figure 4-26 (left): Adapting the electron dot structure of butane to make butan-1-ol. One of the hydrogen atoms on the leftmost carbon is replaced by a hydroxyl (OH) fragment with seven valence electrons. This completes the octets of the carbon and oxygen.
Figure 4-27 (right): The structure of butan-1-ol can be adapted to make that of butanoic acid; the two hydrogen atoms on carbon 1 are replaced by a single oxygen atom via a double bond. |
Drawing electron dot structures of carboxylic acids is a bit more involved but introduces no new concepts. Recall that butanoic acid has a carbonyl functionality on the same carbon that bears the hydroxyl group. We can adapt our structure of butanol by removing the two hydrogen atoms on the first carbon; this frees up two electrons that can form a double bond to a neutral oxygen atom (Figure 4-27). Note that the valences of all the atoms in our final structure for butanoic acid are as predicted. We need to emphasize at this point that the “removal” of the two hydrogen is a formalism only, we are not implying any actual chemical reaction here. Our goal is to have you get comfortable drawing and modifying Lewis structures and part of that skill is getting accustomed to the “tinker toy” approach to valence. We can dissemble and assemble molecules to our hearts’ content, building new ones with the fragments. There is no guarantee that we will come up with a viable structure, as our earlier example of H2O5 attests, but if we limit ourselves to known functional groups, the odds are good that our new structures will indeed be plausible.
The above approach to building organic molecules provides us with a good opportunity to introduce some new functional groups. We've already done so above when we introduced the alkynes, and the above example, where we modified the structure of butane to make an alcohol, can be applied in other ways. The hydroxyl group, OH, has seven valence electrons, as we've seen. It completes its octet by making a single bond to a molecular fragment with an unpaired electron; we used the n-butyl group above to build butan-1-ol. Halogen atoms like fluorine, chlorine, bromine, and iodine, also have seven valence electrons. All of these isoelectronic species satisfy their octets by similarly making one bond with another molecular fragment with an incomplete octet. We can therefore replace a hydrogen atom on any alkane with a halogen atom to make an alkyl halide, often abbreviate RX, where X signifies either F, Cl, Br or I. Using the same alkyl group as above, we can generate structures for 1-fluorobutane, 1-chlorobutane, 1-bromobutane and 1-iodobutane, which are all known and stable compounds. You should be able to see that such an approach allows you to see many other possibilities. For example, how many isomers of 1-chlorobutane exist? Because we generated this compound by replacing a hydrogen on the first carbon, we can look at other possible sites for such a replacement. There is only one: we can replace a hydrogen on the second carbon with a chlorine and get 2-chlorobutane. Prove to yourself that "3-chlorobutane" and "4-chlorobutane" are really just 2-chlorobutane and 1-chlorobutane (respectively) that are improperly named. As for naming alkyl halides, the rules we laid out in Chapter 1 for naming alkanes are easily modified to include halogen atoms; just indicate the position of the halogen atom along the carbon backbone with a locant and name the substituent either fluoro-, chloro-, bromo-, or iodo, as in the above examples.
4-14. If you start with 2-methylpropane, C4H10, how many isomers of C4H9Cl can you generate by replacing one hydrogen atom with a chlorine atom?
Solution
There are two possible products, shown below.
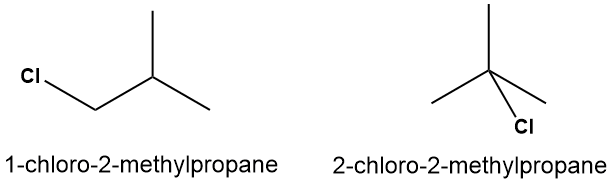
It might be helpful to review the section of "equivalent hydrogen atoms" at this point (see Figure 1-18). 2-methylpropane only has two sets of equivalent hydrogen atoms: the 9 hydrogen atoms that are part of the three -CH3 groups, and the lone hydrogen atom on the central carbon. If we are replacing just one hydrogen atom, we can choose either one from the set of 9, or only hydrogen from from the set of 1. No other possibilities exist.
4-15. If you start with 1-chloro-2-methylpropane and replace a hydrogen atom with a second chlorine atom you would get a compound with the formula C4H8Cl2. Draw and name all the possible isomers that share this molecular formula. (Hint: there are 3 isomers).
We close this section with the introduction of two additional functional groups: ethers and amines. In the above examples, we replaced hydrogen atoms of alkanes with a hydroxyl to form an alcohol, or halogen atoms to form alkyl halides. We can use a similar approach and replace hydrogen atoms from compounds other than alkanes too. For example, if we started with water and replaced a hydrogen atom with a methyl group, what would we get? An alcohol again, in this case methanol (Figure 4-28). Alcohols can therefore be viewed as derivatives of alkanes or water, it just depends on what you use as the starting point for the replacement. But what if we repeated the above replacement, that is, what if we replaced both hydrogen atoms of water with methyl groups? What would we get then? It wouldn't be an alcohol because that is defined by the presence of an -OH functional group. No, the resulting product if you replaced both hydrogen atoms with alkyl groups is called an ether, often abbreviated R-O-R (or R-O-R' because the alkyl groups do not need to be the same. [23]
Figure 4-28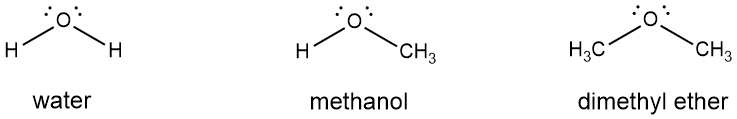 . The stepwise replacement of hydrogen atoms of a molecule of water with methyl groups; replacement of one hydrogen atom gives an alcohol, replacement of both yields an ether.
. The stepwise replacement of hydrogen atoms of a molecule of water with methyl groups; replacement of one hydrogen atom gives an alcohol, replacement of both yields an ether.
We have not written too much about ammonia, NH3, yet but it is an extremely important compound in its own right, from biological, environmental, and economic perspectives [24]. It is a binary compound of nitrogen and hydrogen. Just as the hydrogen atoms of water can be replaced with alkyl groups, the hydrogen atoms of ammonia can be similarly replaced. The resulting compounds are all called amines, and they subdivided into different groups by specifying the number of alkyl groups are bonded to the nitrogen atoms: primary (denoted as 1°), secondary (2°), and tertiary (3°) amines have 1, 2, and 3 alkyl groups, respectively (Figure 4-29). As is the case with ethers, the alkyl groups bonded to the nitrogen atom do not need to be the same; we'll postpone a discussion of amine nomenclature until we study the chemistry of these compounds in greater detail.
 Figure 4-29. (top) The electron dot structure of ammonia, explicitly showing the octet of electrons around the central nitrogen; (bottom) the stepwise replacement of hydrogen atoms of a molecule of ammonia with methyl groups; sequential replacement of hydrogen atom yields methylamine, dimethylamine, and trimethylamine, which are primary, secondary and tertiary amines, respectively.
Figure 4-29. (top) The electron dot structure of ammonia, explicitly showing the octet of electrons around the central nitrogen; (bottom) the stepwise replacement of hydrogen atoms of a molecule of ammonia with methyl groups; sequential replacement of hydrogen atom yields methylamine, dimethylamine, and trimethylamine, which are primary, secondary and tertiary amines, respectively.
Footnotes and References.
[22] The term homodiatomic refers to molecule consisting of two atoms of the same element.
[23] Time out for a really bad chemistry joke.
Q: Why are ethers (R-O-R) called ethers?
A: Because ether way you write it, it's the same!
[24] More than 200 million tons of ammonia is produced globally every year, primarily for fertilizers. The compound quite literally makes it possible to feed billions of people around the world.

