4.6: Interpreting Electron Dot Structures
- Page ID
- 417763
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
When considering how a given molecules reacts or otherwise behaves, a chemist's first instinct is usually to draw its electron dot structure. The practice isn't just to keep track of the species are involved, like a manager reviewing the lineup before a baseball game, because the drawings themselves can provide a surprising amount of insight into the actual structure of molecules which, in turn, can suggest modes of reactivity. We touched on this when we discussed linoleic acid back in Chapter 1, where we pointed out the kinks in the backbone and its tendency to react with oxygen and thereby "spoil". Both effects are due to the presence of double bonds and these are immediately visible from the line diagrams we drew, and line diagrams are just stylized and uncluttered versions of electron dot structures.
In this section we describe two important structural characteristics of molecules that can be gleaned from electron dot structures (Figure 4-30):
- Molecular geometry, which refers to bond angles, defined as the angle between two atoms that are bonded to a third, central, atom. It may be helpful to point out at the outset, however, that the term "molecular geometry" is somewhat of a misnomer, as it refers to the geometry around a specific atom in a molecule, and not necessarily to the molecule as a whole.
- Relative bond lengths, defined as the distance between the nuclei of two atoms joined by a covalent bond.
 Figure 4-30. The two structural features that can be gleaned from electron dot structures. The figure shows three atoms, E, X and Y, joined by covalent between bonds: the bond angle (red), is defined as the angle formed between atomic nuclei of three contiguous atoms, and bond lengths (blue), are the distances between nuclei of adjacent atoms.
Figure 4-30. The two structural features that can be gleaned from electron dot structures. The figure shows three atoms, E, X and Y, joined by covalent between bonds: the bond angle (red), is defined as the angle formed between atomic nuclei of three contiguous atoms, and bond lengths (blue), are the distances between nuclei of adjacent atoms.
We'll start with molecular geometry because we've made numerous references to the shapes of molecules to this point. The approach we outline below is both exceptionally powerful and very simple (how often does that happen?). It is powerful in the sense that you can predict, often to within just a couple of degrees, the angles between atoms for nearly every molecule of biological significance – this includes the angles assumed by atoms in proteins, carbohydrates, lipids, and nucleic acids – as well as smaller molecules such as solvents, most organic structures, and inorganic polyatomic ions. It is simple in the sense that all you need to make those predictions is a valid electron dot structure.
To estimate bond angles around a given atom in an electron dot structure we use a model called Valence Shell Electron Pair Repulsion, or VSEPR. The basic rationale is pretty simple: pairs of electrons, whether they are shared with another atom in a covalent bond or are unshared lone pairs, repel each other and are situated as far away from other electron pairs as possible. The resulting geometries are a consequence of this repulsion. As the logic of this approach would dictate, the predicted angles are directly related to how many "sets" of electrons are repelling each other. This is given by its steric number, defined as the number of lone pairs of electrons it has plus the number of other atoms it is bonded to. Be careful here: for most organic molecules, you might expect there to be four sets of electrons because electrons are usually paired and the octet rule says there should be 8 total on a given atom of carbon, nitrogen or oxygen, hence four pairs. This is an incorrect interpretation of how steric number is defined. It might be counterintuitive, but all electrons in a multiple bond are considered as a single "set" of electrons for the purposes of using VSEPR, so there are many occasions where carbon has a steric number of less than 4. This is because all of electrons in multiple bonds are directed at a single atom and do not mutually repel each other in the same way as electrons bonding to other atoms or lone pairs do.
Problem 4-16: What is the steric number of the carbon atoms in each of the following structures? What about the oxygen atom in question b?

Solutions
- Carbon has a steric number of 3. Explanation: the carbon atom has zero lone pairs and is bound to three atoms.
- Carbon has a steric number of 4; the oxygen atom has a steric number of 4 as well because it has two lone pairs and is bonded to two atoms.
- Carbon has a steric number of 2.
- The compound is but-1-ene (or 1-butene) and has four carbon atoms. Carbon atoms numbered one and two (those connected by the double bond) have steric numbers of 3, while carbon atoms three and four have steric numbers of 4.
While it is possible to use VSEPR to estimate bond angles and geometries for atoms bonded to as many as 6 other atoms, we will focus on atoms bonded to 2, 3 or 4 other atoms because this covers virtually all organic and biochemically important molecules. Note that you can't apply the concept of bond angles for atoms bonded to only one other atoms because, by definition, you need three points to define an angle. Thus you can't define any molecular geometries for the oxygen atom in Problem 4-16a, the nitrogen atom of 4-16c, or any of the hydrogen atoms in any of the examples in that question because they each is attached to only one other atom and therefore cannot be the vertex of an angle.
The ideal angles formed by atoms with steric numbers 2 through 4 are shown in Figure 4-31. In the diagram, lines originating from the central atom E represent the directions of the "sets of electrons" for the steric numbers shown. In each case, the lines are positioned to maximize the distance between them. When there are only two, they are oriented 180° away from each other. When there are three lines, they are positioned 120° from each other and, this is important, they lie in the same two-dimensional plane. When we have four lines, representing four sets of electrons mutually repelling each other, they must be oriented in three-dimensions and all of them are 109° from the other three other. This is represented in the figure by having one line "coming out of the page", represented by the hashed wedge at the bottom left, and another line going behind the page, represented by the faint gray line. The other two lines are in the plane of the page. If there were objects at the end of each of these four lines, they would occupy the vertices of a perfect tetrahedron, with E in the exact center of it.
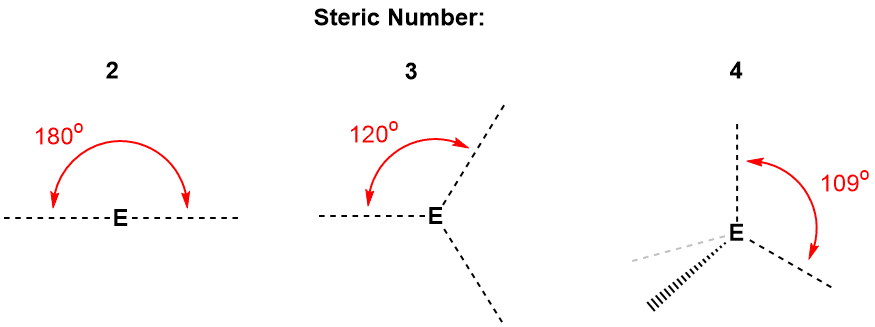 Figure 4-31. The orientation of sets of electrons for atoms with steric numbers of 2 (left), 3 (center) and 4 (right). For atoms with a steric number of 2, the sets of electron are positioned 180° apart from each other; For atoms with a steric number of 3, sets of electrons are oriented 120° to each other and are centered in the same 2-dimensional plane; For atoms with a steric number of 4, the sets of electrons are positioned 109° from each other and are directed at the vertices of a tetrahedron. These geometries are the ideal VSEPR bond angles and illustrate the electron pair geometry corresponding to these steric numbers.
Figure 4-31. The orientation of sets of electrons for atoms with steric numbers of 2 (left), 3 (center) and 4 (right). For atoms with a steric number of 2, the sets of electron are positioned 180° apart from each other; For atoms with a steric number of 3, sets of electrons are oriented 120° to each other and are centered in the same 2-dimensional plane; For atoms with a steric number of 4, the sets of electrons are positioned 109° from each other and are directed at the vertices of a tetrahedron. These geometries are the ideal VSEPR bond angles and illustrate the electron pair geometry corresponding to these steric numbers.
VSEPR makes use of the orientation of sets of electrons as depicted in Figure 4-31. To illustrate how VSEPR is generally applied, we'll go through a series of compounds, all having a steric number of 4 on the central atom but which have different numbers of lone pairs on the central atom, specifically CH4, NH3, and H2O, which have 0, 1 and 2 lone pairs of electrons, respectively (Table 4-2). The central atoms of these molecules all share the same electron pair geometry, which is the geometry assumed by their respective the sets of electrons. In all cases, the electron pairs, whether they are bonding or non-bonding, are oriented roughly 109° from each other and are oriented at the vertices of a tetrahedron. They therefore correspond to the right-hand drawing in Figure 4-31.
| compound | methane (CH4) | ammonia (NH3) | water (H2O) |
|---|---|---|---|
| electron dot structure | 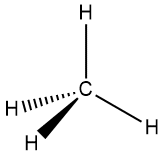 |
 |
 |
| ball and stick model | 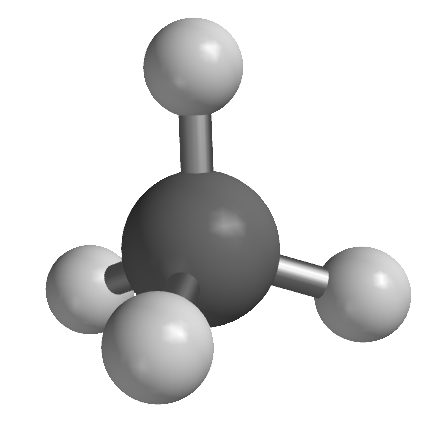 |
 |
 |
| electron pair geometry | tetrahedral | tetrahedral | tetrahedral |
| molecular geometry | tetrahedral | pyramidal | angular |
| ideal bond angle | 109.5° | 109.5° | 109.5° |
| observed bond angle | 109.5° | 107.8° | 104.5° |
The entries in Table 4-2 serve as a model for how VSEPR is generally used, so we discuss some aspects of the three cases below as they will help develop your understanding of how to apply the approach to other molecules. First, as methane illustrates, when there are no lone pairs on the central atom, the electron pair geometry is the same as the molecular geometry. In this case both are tetrahedral. This is an example of a general rule that we will see again for steric numbers of 2 and 3. And because all four atoms bound to the central atom are the same, the observed bond angles match perfectly with that predicted by VSEPR: 109.5°. Second, when one lone pair replaces one of the atoms bound to the central one, as in ammonia, the steric number and electron pair geometry remain the same – namely, 4 and tetrahedral, respectively – but the molecular geometry changes. Because the mass of the electron cloud of the lone pair on the nitrogen atom is miniscule compared to the rest of the molecule, it is treated as if it were invisible. A comparison of the ball and stick models of methane and ammonia in the table serves to emphasize this point: if the central atoms were the same color, the only major change in going from methane to ammonia would be the "disappearance" of the topmost hydrogen. In the case of ammonia, the shape of the molecule can be described as a nitrogen atom sitting atop a three-sided pyramid (albeit a short one!) and the molecular geometry is therefore described as pyramidal. Also note that there is a minor deviation from the ideal bond angle: the H-N-H bond angles in ammonia are 107.8, within two degrees of the ideal angle predicted by VSEPR.
When two lone pairs replace two atoms, as we see with water compared to methane, only three atoms are now "visible" and the molecular geometry is called angular or bent. As we saw with ammonia, there is a slight deviation from the ideal bond angle: the H-O-H bond angle is five degrees smaller in water than VSEPR would predict. Both deviations can be rationalized by the fact that nonbonding electron pairs are not directed toward a specific point in space – they are more diffuse than bonding pairs, in other words. As such, they tend to spread out more and repel bonding pairs in such a way that the latter are slightly squeezed together, decreasing the bond angles. Because water has two non-bonding pairs of electrons, the deviation is slightly larger than that seen in ammonia, which has one. These minor deviations are common and need not unduly concern you because they rarely, if ever, have a significant effect on the properties or reactivities of molecules.
The same sort of discussion can be developed to explain the molecular geometries of molecules with central atoms having steric numbers of 2 and 3 (Table 4-3). For a steric number of 2 we only need to consider cases where there is no lone pair on the central atom (why?). As shown in Figure 4-31, to maximize the separation between two sets of electrons, they should be positioned 180° apart. The electron pair geometry is therefore described as linear, as is the molecular geometry. The predicted bond angle of 180° exactly matches the observed geometry of carbon dioxide, CO2. The same geometry is seen in the triply-bonded carbon atoms in alkynes for the same reason: carbon atoms that have a triple bond will have a steric number of 2. For steric numbers of 3, there are two molecular geometries that can arise from the trigonal planar electron pair geometry that results from having three sets of electrons mutually repel each other. When there are no lone pairs, the molecular geometry is trigonal planar, matching that of the electron pair geometry. This is the geometry assumed by carbon atoms that have one double bond and two single bonds, as seen with formaldehyde in Table 4-3, as well as in alkenes, carboxylic acids and some other functional groups. The ideal bond angle for a trigonal planar geometry is 120°, but that is only perfectly met when all three atoms bound to the central atom are the same. The observed bond angles in most organic compounds are usually within few degrees of this ideal; the bond angles observed in formaldehyde are typical in that regard.
For molecules that have a central atom with a steric number of 3 and one lone pair, the electron pair geometry is once again trigonal planar, but the molecular geometry is angular or bent, the same terms used for atoms with a steric number of 4 with 2 lone pairs, like water. The example shown in Table 4-3 is ozone, the allotrope of oxygen with the formula O3. The electron dot structure for ozone is peculiar in that two of the three oxygen atoms do not have 2 bonds, and we will discuss some aspects of the bonding of this molecule in the next section. For the time being, if you accept the electron dot structure shown (all three oxygen atoms obey the octet rule, so this is not an absurd request!), then you can see how the steric number of the central atom is indeed 3. The expected bond angle is 120°, close to the observed value of 117°.
| Steric Number: 2 | Steric Number: 3 | ||
|---|---|---|---|
| compound | carbon dioxide (CO2) | formaldehyde (CH2O) | ozone (O3) |
| electron dot structure |  |
 |
 |
| ball and stick model | 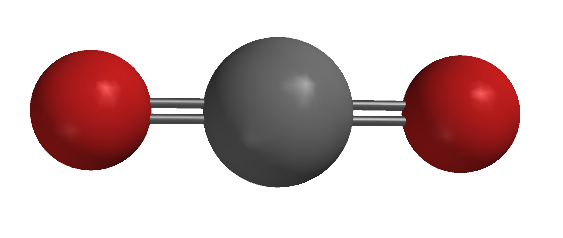 |
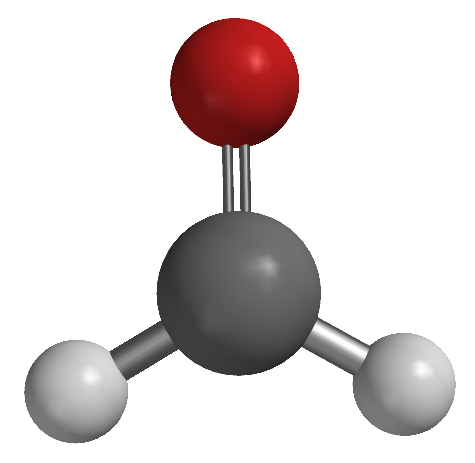 |
 |
| electron pair geometry | linear | trigonal planar | trigonal planar |
| molecular geometry | linear | trigonal planar | angular |
| ideal bond angle | 180° | 120° | 120° |
| observed bond angle | 180° | H-C-H: 118° O-C-H: 121° |
117° |
At a Glance: Applying VSEPR
The examples shown in Tables 4-2 and 4-3 exemplify for VSEPR is used for nearly all organic molecules and those of biochemical interest. In summary, to predict the geometry of an atom in any molecule you:
1. Start with a valid electron dot structure (or line structure - but be careful of hidden lone pairs on atoms like oxygen and nitrogen!)
2. Use the steric number and number of lone pairs to determine the corresponding molecular geometry. Table 4-4 summarizes the possible molecular geometries for steric numbers 2, 3 and 4.
| Steric Number of Central Atom | Number of Lone Pairs on Central Atom | Angle Between Electron Pairs | Electron Pair Geometry | Molecular Geometry | Example |
|---|---|---|---|---|---|
| 2 | 0 | 180° | linear | linear | CO2, HCN |
| 3 | 0 | 120° | trigonal planar | trigonal planar | CH2O |
| 1 | 120° | trigonal planar | angular | O3 | |
| 4 | 0 | 109° | tetrahedral | tetrahedral | CH4 |
| 1 | 109° | tetrahedral | pyramidal | NH3 | |
| 2 | 109° | tetrahedral | angular | H2O |
Problem 4-17. Describe the molecular geometry of hydrogen cyanide, HCN.
Solution
We must first determine the steric number of the central atom and, to do that, we need the the electron dot structure, shown below.
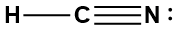
The steric number of carbon is 2 because it is bound to two other atoms and has no lone pairs. The electron pair geometry and the molecular geometry are therefore linear.
Problem 4-18. Amines often have strong, unpleasant odors. Ammonia, the simplest amine, is a case in point. The structure below represents an amine that goes by the common name of cadaverine and is, as you may surmise, produced by decaying flesh and partially gives rise to the associated foul odor of dead animals.
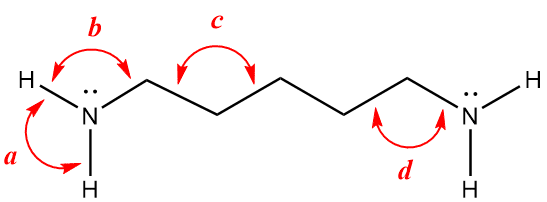
Using VSEPR, estimate the angles labeled a-d in the above figure, representing H-N-H, H-N-C, C-C-C, and C-C-N bond angles, respectively. To help you out, the lone pairs of the nitrogen atoms are written explicitly. Also note that all carbon atoms obey the octet rule, so make sure you account for all hydrogen atoms, whether they are drawn or not. Finally, don't let the 2-dimensional nature of the figure confuse you; bond angles of three-dimensional objects can be greatly distorted by two-dimensional representations. Apply the basic principles of VSEPR and don't second guess yourself based on the appearance of the line structure.
Problem 4-19. Here's a more pleasant smelling compound: ß-damascone, which is partially responsible for the scent of roses. It has the molecular formula C13H20O. Based on VSEPR, how many of the carbon atoms will have tetrahedral geometries? How many will be trigonal planar? How many will be linear?

We started this section stating that there were two structural features that are readily determined from electron dot structures: molecular geometry and relative bond lengths. We now turn our attention to the latter. Here's the short version: for bonds between any two atoms, X and Y, the X-Y bond length increases in the order: single > double > triple. In other words, "other things being equal", single bonds are longer than double bonds, which are longer than triple bonds. What "other things" might be relevant to warrant the use of that guarded phrase, "other things being equal"? Although we have not discussed the relative sizes of atoms, it probably won't come as a surprise that atoms of different elements have different sizes. Hydrogen atoms are small compared to carbon and oxygen, for example. As a result the bond lengths between hydrogen and other atoms tends to be smaller than for bond lengths between other atoms. By restricting the comparison to atoms of the same two elements, X and Y, we remove the complication that varying atomic radii would otherwise introduce.
It is useful here to define a term - bond order - which refers to the "single" in single bonds, "double" in double bonds and "triple" in triple bonds. It is equal to the number of pairs of electrons in comprising a given covalent bond. Single bonds have a bond order of 1, double bonds have a bond order of 2, and, predictably, triple bonds have a bond order of 3. Thus the higher the bond order between two atoms, the shorter the bond between those atoms. This reflects the fact that double bonds are considerably stronger than single bonds between the same atoms, and triple bonds are stronger than double bonds. You can rationalize this the following way: nuclei, which are positively charged, are held in close proximity to each other by their mutual attraction to the negatively electrons of the shared electrons of the covalent bond; double and triple bonds have more electrons in the space between the nuclei, which pulls them closer together owing to the stronger electrostatic attraction. Because electron dot structure reveal bond orders explicitly, they can therefore provide insights into bond lengths between atoms across similar series, at least relatively. Three sets of examples of this pattern are shown the top of Figure 4-32. Shown are molecules between atoms of the came elements (carbon, nitrogen, and oxygen) having single, double, and triple bonds (although there is no entry for a molecule containing and oxygen-oxygen triple bond - why not?). The correlation between bond order and bond length can also be shown graphically (Figure 4-32, bottom). While the trend is not perfectly linear, the obvious correlation provides evidence that the electron dot diagrams reflect tangible structural features of the molecules they represent.

Figure 4-32. (Top) Trends seen in bond lengths for compound with carbon-carbon, nitrogen-nitrogen and oxygen-oxygen covalent bonds. The carbon-containing compounds are, from left to right: ethane, ethene, and ethyne; the nitrogen compounds are hydrazine (a rocket propellant), diazine, and dinitrogen; the oxygen compounds are hydrogen peroxide and dioxygen, Bond distances (in pm) are shown above the electron dot structures. Bonds get progressively shorter as the bond order increases. 1 pm = 1 \( \times \) 10-12 m.
(Bottom) Bond distances as a function of bond order; note that the trends are consistent for the three sets compounds but they are offset from each other because of differences in atomic radii.

Problem 4-20. Electron dot structures of three compounds containing carbon-oxygen bonds are shown at right. The measured bond lengths in these compounds are 112, 123, and 143 pm. Match the bond lengths to the appropriate structure.
Problems
Problem 4-21. The electron dot diagram of acetic acid is shown below. State the electron pair geometries and molecular geometries of all atoms for which bond angles can be defined, that is, for all atoms bonded to more than one other atom. (Note, no lone pairs are shown, but all carbon and oxygen atoms obey the octet rule.)
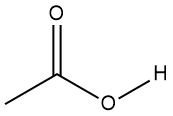
Problem 4-22. Referring again to the structure of acetic acid, predict which carbon-oxygen bond is the longest.
Problem 4-23. Problem 4-19 concerned one of the compounds responsible for the lovely scent of freshly cut roses. Two other compounds that contribute to the aroma are shown below (hat tip: the always fascinating Compound Interest). Both feature six-membered rings and all atoms (except hydrogen) obey the octet rule.

a) Determine the steric numbers for all the carbon atoms in both structure. Determine the steric number for the oxygen atoms.
b) Based only on VSEPR considerations, estimate the bond angles that would be observed inside of the rings, labeled a and b above.
c) Estimate the C-O-H and C-O-C bond angles, labeled c and d above.
d) Estimate the C-C-C bond angle labeled e above.
3) Which carbon-carbon bond in the right-hand structure is the shortest?
Problem 4-24. Critique the following statement. "Because C-H bonds all have a bond order of 1, they are expected to be longer than carbon-oxygen double bonds."
Problem 4-25. Consider the following series of compounds containing carbon-nitrogen bonds (hydrogen cyanide is on the left, methylamine is on the right and, in the center, is an example of a compound known as an imine, defined as having a carbon-nitrogen double. Estimate the H-C-N bond angle for each one and rank the carbon-nitrogen bonds from shortest to longest. What are the molecular geometries for the carbon atoms in each structure? Are molecular geometries applicable to the nitrogen atoms in all of the compounds? Why or why not? Give the molecular geometries for all the nitrogen atoms for which the term is meaningful.

Problem 4-26. To illustrate the effect of the number of lone pairs on molecular geometry, Table 4-2 included compounds having central atom with the same steric number (4), but different number of lone pairs (0, 1, and 2). HF is a molecule in the fluorine has a steric number of 4 and has 3 lone pairs. Why wasn't it included in the table?
Problem 4-27. Based on the data shown in the graph at the bottom of Figure 4-32, which element do you think has the larger atomic radius, carbon or nitrogen? Explain.
 Problem 4-28. The phosphate ion, PO43-, plays a large role in many biochemical systems. Its electron dot structure is shown at right. As you can see, the phosphorus atom exhibits an "expanded" octet, having five covalent bonds and therefore ten electrons. There are no lone pairs on the phosphorus, but each oxygen has enough lone pairs (not shown) to satisfy their octets. Based on VSEPR considerations, state the steric number of the phosphorus atom, its electron pair geometry, the molecular geometry of the ion, and estimate the O-P-O bond angles.
Problem 4-28. The phosphate ion, PO43-, plays a large role in many biochemical systems. Its electron dot structure is shown at right. As you can see, the phosphorus atom exhibits an "expanded" octet, having five covalent bonds and therefore ten electrons. There are no lone pairs on the phosphorus, but each oxygen has enough lone pairs (not shown) to satisfy their octets. Based on VSEPR considerations, state the steric number of the phosphorus atom, its electron pair geometry, the molecular geometry of the ion, and estimate the O-P-O bond angles.

