13.6: Polarity and Properties
- Page ID
- 357689
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We saw in the previous section that molecules can be classified as polar or nonpolar, depending on the types of bonds present in the molecule and its overall molecular geometry. The major difference between the two types of molecules was the presence of partially positive and partially negative regions in the polar molecule. These charged regions will cause polar molecules to have very different properties than nonpolar molecules.
Properties of Polar Molecules
Polar molecules tend to align themselves when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure \(\PageIndex{1}\)). We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted.
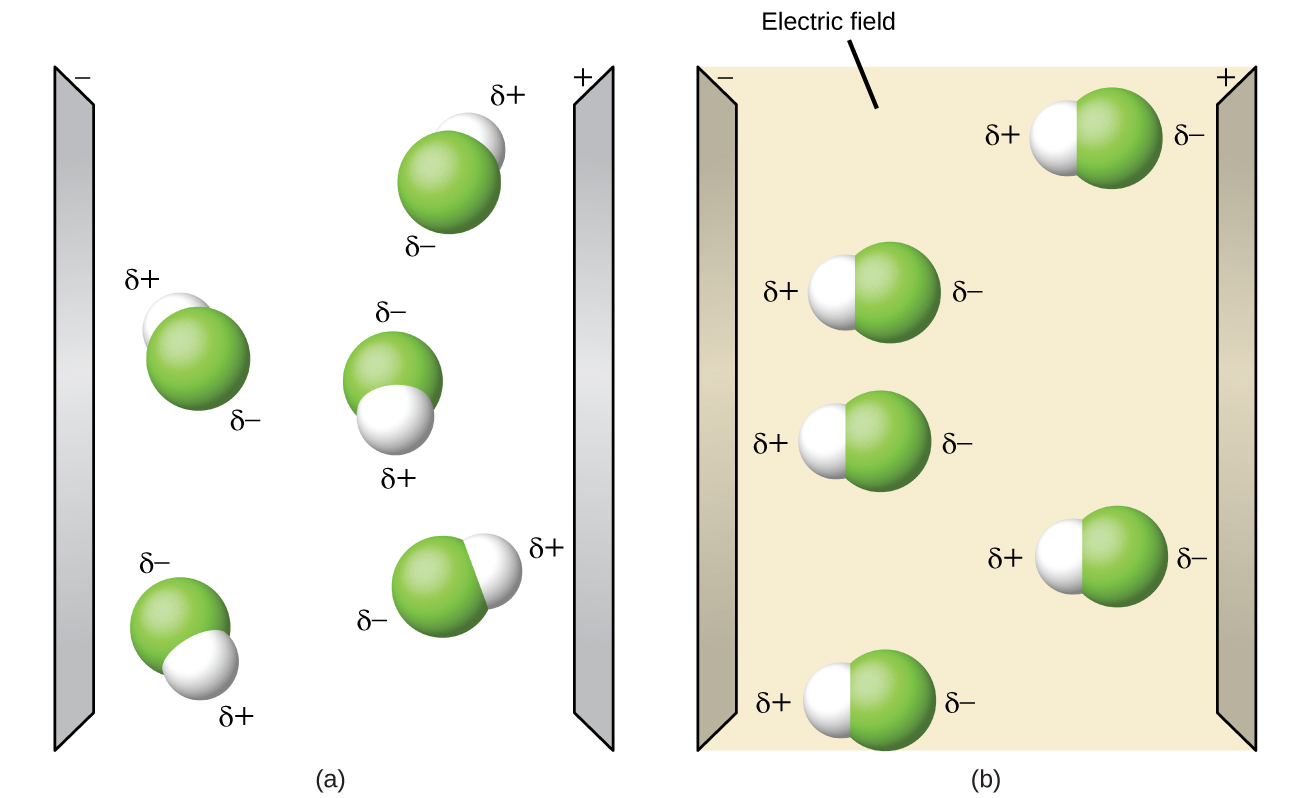
In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be attracted to the positive and negative regions of neighboring polar molecules. Because of this, polar molecules tend to:
- have higher melting points than nonpolar molecules
- have higher boiling points than nonpolar molecules
- be more soluble in water (dissolve better) than nonpolar molecules
- have lower vapor pressures than nonpolar molecules
It should be noted that while molecules can be described as polar, this is often a relative term, with one molecule simply being more polar or less polar than another.
Like Dissolves Like
A simple way to predict which compounds will dissolve in other compounds is the phrase "like dissolves like." What this means is that substances comprised of nonpolar molecules tend to dissolve other substances comprised of nonpolar molecules. Substances comprised of polar molecules tend to dissolve other substances comprised of polar molecules. In addition, substances comprised of highly polar molecules, like water, often dissolve ionic compounds. Again, this can be loosely attributed to the presence or absence of charged regions in the molecule. Polar molecules will be attracted to the charged regions in other polar molecules, causing them to interact with each other and form a solution. In contrast, when polar and nonpolar molecules are mixed together, the lack of charged regions in the nonpolar molecules will cause the polar molecules to only be attracted to themselves, excluding the nonpolar molecules and creating two distinct regions instead of a solution. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a solution.
Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Have you ever wondered why fish are able to breathe? Oxygen gas, a nonpolar molecule, dissolves to a small degree in water – it is this oxygen that the fish take in through their gills. The reason we can enjoy carbonated sodas is also due to a nonpolar compound that dissolves in water. 7-Up and all other sodas have carbon dioxide gas, CO2, a nonpolar compound, dissolved in a sugar-water solution. In this case, to keep as much gas in solution as possible, the sodas are kept under pressure.
This concept of "like dissolves like" is summarized in Table \(\PageIndex{1}\):
| Polarity of Solute | Polarity of Solvent | Dominant Intermolecular Force | Is Solution Formed? |
|---|---|---|---|
| polar | polar | dipole-dipole force and/or hydrogen bond | yes |
| nonpolar | nonpolar | dispersion force | yes |
| polar | nonpolar | not applicable | no |
| nonpolar | polar | not applicable | no |
| ionic | polar | ion-dipole | usually |
| ionic | nonpolar | not applicable | no |
A more precise explanation for what makes a solute soluble in some solvents, but not others, lies in intermolecular interactions. The intermolecular interactions include dispersion forces, dipole-dipole interactions, and hydrogen bonding (as described in the next section). From experimental studies, it has been determined that if molecules of a solute experience similar intermolecular forces to the solvent, the solute will likely dissolve in that solvent. So, NaCl, an ionic compound, dissolves in water since the ions are able to interact with the partially charged regions of polar water molecules, but not in oil, which is generally nonpolar. Nonpolar wax dissolves in nonpolar hexane, but not in polar water.

✅ Example \(\PageIndex{1}\): Polar and Nonpolar Solvents
Would I2 be more soluble in CCl4 or H2O? Explain your answer. If necessary, manipulate their molecular models below.
Solution
I2 is nonpolar. Of the two solvents, CCl4 is nonpolar and H2O is polar, so I2 would be expected to be more soluble in CCl4.
✏️ Exercise \(\PageIndex{1}\)
Would HCl (see the molecular model below) be more soluble in CCl4 or H2O? Explain your answer.
- Answer
-
H2O, because HCl and H2O are both polar molecules. CCl4 is a nonpolar molecule.
✅ Example \(\PageIndex{2}\)
Water is considered a polar solvent. Which substances should dissolve in water?
- methanol (CH3OH), shown below
- sodium sulfate (Na2SO4)
- octane (C8H18), shown below
Solution
Because water is polar, substances that are polar or ionic will dissolve in it.
- Because of the shape of the molecule and the polar —OH grouping in methanol, we expect its molecules to be polar and for it to be soluble in water. As both water and methanol are liquids, the word miscible can be used in place of soluble.
- Sodium sulfate is an ionic compound, so we expect it to be soluble in water.
- Like other hydrocarbons, octane is nonpolar, so we expect that it would not be soluble in water.
✏️ Exercise \(\PageIndex{2}\)
Toluene, C6H5CH3, (see the molecular model below) is widely used in industry as a nonpolar solvent. Which substances should dissolve in toluene?
- water (H2O)
- sodium sulfate (Na2SO4)
- octane (C8H18)
- Answer
-
Water (H2O) is polar, sodium sulfate (Na2SO4) is ionic, and octane (C8H18) is nonpolar. Therefore, octane is the only substance that should dissolve in toluene.
Summary
- Polar molecules tend to have higher melting points and boiling points than nonpolar molecules.
- “Like dissolves like” is a useful rule for deciding if a solute will be soluble in a solvent.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by StackExchange (thomij), Melissa Alviar-Agnew, Henry Agnew, Lance S. Lund (Anoka-Ramsey Community College), and Vicki MacMurdo (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.



