14.2: Solution Terminology
- Page ID
- 289453
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Learn terminology involving solutions.
- Qualitatively explain the amount of solute dissolved in a solvent.
A solution is a homogenous mixture. The component of a solution that does the dissolving is called the solvent, while the component that gets dissolved is called the solute. Almost always, the solvent is the component that is present in the greatest proportion by mass, moles, or volume.
When one substance dissolves into another substance, we say it is soluble in that substance. If a substance does not dissolve into another substance, we say it is insoluble in that substance. When two liquids can dissolve into each other in any proportion, we say the liquids are miscible with each other. If they cannot dissolve into each other, we say they are immiscible with each other.

Solutions exist for every possible phase combination of solute and solvent. Salt water, for example, is a solution of solid NaCl in liquid water, while air is a solution of a gaseous solute (O2) in a gaseous solvent (N2). In all cases, however, the overall phase of the solution is the same phase as the solvent. A solution in which the overall phase is a gas is called a gaseous solution. A solution in which the overall phase is a liquid is called a liquid solution. A solution in which the overall phase is a solid is called a solid solution. Table \(\PageIndex{1}\) lists some common types of solutions, with examples of each.
As we have just learned, a solution need not involve water. However, when water is the solvent, it is called an aqueous solution. When the solvent is any substance other than water, it is called a nonaqueous solution.
In many cases, the relative amounts of solute and solvent may be referenced using the terms dilute and concentrated. A dilute solution is one that contains a relatively small amount of solute dissolved in a solvent, while a concentrated solution is one that contains a relatively large amount of solute dissolved in a solvent. There is no set cut-off point that distinguishes a dilute solution from a concentrated solution and what constitutes dilute or concentrated may vary with the solute and/or solvent.
Solubility and Saturation
Table salt, NaCl, readily dissolves in water. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is specified as the solubility of the solute. It is usually expressed in terms of the amount of solute that can dissolve in 100 g of the solvent at a given temperature. Table \(\PageIndex{2}\) lists the solubilities of some simple ionic compounds at 25°C. These solubilities vary widely. NaCl can dissolve up to 36.1 g per 100 g of H2O at 25°C, while AgCl can dissolve only 0.00019 g per 100 g of H2O at 25°C.
| Solute | Solubility (g per 100 g of H2O at 25°C) |
|---|---|
| AgCl | 0.00019 |
| CaCO3 | 0.0006 |
| KBr | 70.7 |
| NaCl | 36.1 |
| NaNO3 | 94.6 |
When the maximum amount of solute has been dissolved in a given amount of solvent at a specified temperature, we say that the solution is saturated with solute. When less than the maximum amount of solute is dissolved in a given amount of solvent at a specified temperature, the solution is unsaturated. These terms are also qualitative terms because each solute has its own solubility. A solution of 0.00019 g of AgCl per 100 g of H2O may be saturated at 25°C, but with so little solute dissolved, it is also rather dilute. A solution of 36.1 g of NaCl in 100 g of H2O at 25°C is also saturated, but rather concentrated. Figure \(\PageIndex{2}\) shows the distinction between an unsaturated solution and a saturated solution of NaCl.
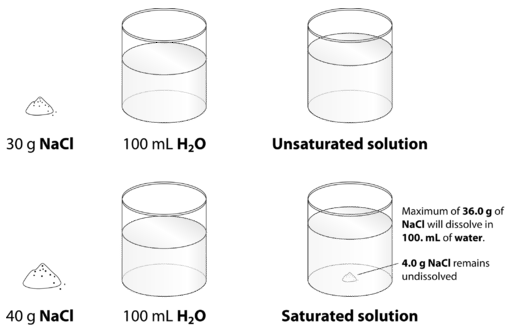
How can you tell if a solution is saturated or unsaturated? If more solute is added and it does not dissolve, then the original solution was saturated. If the added solute dissolves, then the original solution was unsaturated. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated.
This said, it is sometimes possible for a solution to have more solute dissolved than a saturated solution at a given temperature. Such a solution is called a supersaturated solution. A supersaturated solution may be formed by heating the solvent, dissolving more solute than would normally dissolve at regular temperatures, then allowing the solution to slowly cool. Supersaturated solutions are not stable. Given the opportunity (such as dropping a crystal of solute in the solution), excess solute will precipitate from a supersaturated solution.
Video \(\PageIndex{1}\) and Video \(\PageIndex{2}\) show some interesting applications of supersaturated solutions. Video \(\PageIndex{1}\) shows supersaturated sodium acetate solution being used in a reusable hand warmer, while Video \(\PageIndex{2}\) shows how to make rock candy from a supersaturated solution of sucrose.
✅ Example \(\PageIndex{1}\)
A solution is made by dissolving 1.00 g of sucrose, C12H22O11, in 100.0 g of liquid water. Identify the solute and solvent in the resulting solution.
Solution
Either by mass or by moles, the obvious minor component is sucrose, so it is the solute. Water – the majority component – is the solvent. The fact that the resulting solution is the same phase as water also suggests that water is the solvent.
✅ Example \(\PageIndex{2}\)
Indicate whether gasoline and ethanol, CH3CH2OH, are miscible or immiscible with water.
Solution
Gasoline is a mixture of hydrocarbons. Hydrocarbons are nonpolar, while water is polar. Therefore, gasoline and water are immiscible with each other. Ethanol, CH3CH2OH, has a polar —OH grouping that makes ethanol a polar compound and one in which hydrogen bonds exist. Therefore, ethanol and water are miscible with each other.
✏️ Exercise \(\PageIndex{1}\)
A solution is made by dissolving 3.33 g of HCl (g) in 40.0 g of liquid methyl alcohol, CH3OH. Identify the solute and solvent in the resulting solution.
- Answer
-
solute: HCl (g); solvent: CH3OH
✏️ Exercise \(\PageIndex{2}\)
Which solution is an example of an aqueous solution and which is an example of a nonaqueous solution?
- Sugar dissolved in water.
- Naphthalene dissolved in ethanol.
- Answer
-
Sugar dissolved in water is an example of an aqueous solution.
Naphthalene dissolved in ethanol is an example of a nonaqueous solution.
Summary
- Solutions are composed of a solvent (major component) and a solute (minor component).
- Solvents and solutes can be any phase of matter combined together.
- A saturated solution has the maximum amount of solute possible at a given temperature. An unsaturated solution will have less solute than a saturated solution and, in certain cases, a supersaturated solution can be made which has more solute.
This page is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew, Henry Agnew and Lance S. Lund (Anoka-Ramsey Community College).

