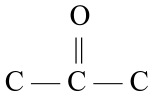12.7: Alcohols, Aldehydes, Carboxylic Acids, and Ketones
- Page ID
- 355092
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alcohols, aldehydes, carboxylic acids, and ketones bear some relation to each other as they are easily converted from one to another through the process of oxidation or reduction. An oxidation reaction is ordinarily thought of as the loss of electrons, while a reduction reaction is ordinarily thought of as the gain of electrons. In organic chemistry, oxidation is typically observed when a molecule gains an oxygen atom (that makes it easy, huh?) and/or loses two hydrogen atoms. Reduction is the opposite process, by which a molecule loses an oxygen atom or gains two hydrogen atoms.
Oxidation of Alcohols
Alcohols may be classified according to the number of carbon atoms directly bonded to the carbon atom that is bonded to the hydroxyl group. If only one carbon atom is directly attached to that carbon, the alcohol is called a primary alcohol. If two carbon atoms are directly attached, the alcohol is called a secondary alcohol. If three carbon atoms are directly attached, the alcohol is called a tertiary alcohol. That's great, but how are alcohols, aldehydes, carboxylic acids, and ketones related?
It turns out that primary alcohols may be oxidized to aldehydes which, in turn, may be oxidized to carboxylic acids. Figure \(\PageIndex{1}\) shows how ethanol, a primary alcohol that has two carbon atoms, is eventually oxidized to ethanoic acid, a two-carbon carboxylic acid. Ethanol is the alcohol found in alcoholic beverages and is produced through the fermentation of sugars and starches in fruits and grains. Ethanoic acid is also called acetic acid, the acid found in vinegar. The oxidation of ethanol is the reason why a bottle of wine will begin to taste sour and take on a vinegary taste several days after it has been opened and exposed to the air.
| Primary Alcohol | Aldehyde |
Carboxylic Acid | ||
 |
\(\xrightarrow{\mathrm{oxidation}}\) | 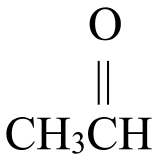 |
\(\xrightarrow{\mathrm{oxidation}}\) | 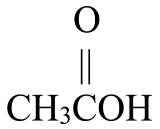 |
| \(\xrightarrow{\mathrm{oxidation}}\) | \(\xrightarrow{\mathrm{oxidation}}\) |
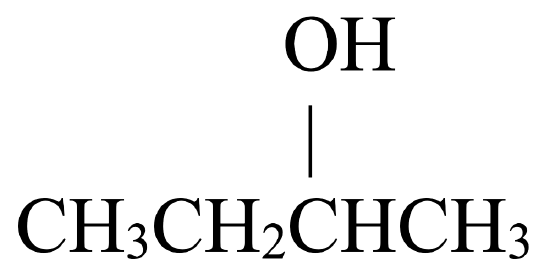
Secondary alcohols, on the other hand, may be oxidized to ketones. Figure \(\PageIndex{2}\) shows how 2-butanol, a secondary alcohol, is oxidized to 2-butanone, a ketone. Notice that the number of carbon atoms does not change, nor does the position to which the function group is attached. 2-butanol is a secondary alcohol because the carbon atom to which the hydroxyl group is attached is directly bonded to two other carbon atoms.
| Secondary Alcohol | Ketone |
|||
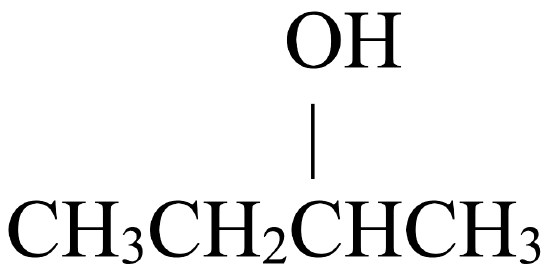 |
\(\xrightarrow{\mathrm{oxidation}}\) | 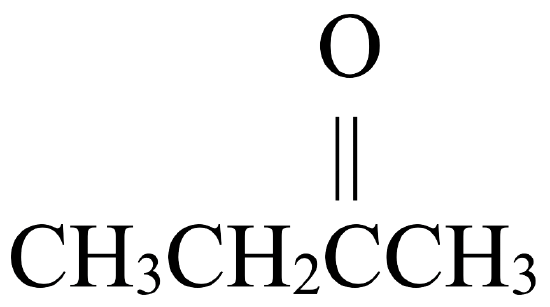 |
||
| \(\xrightarrow{\mathrm{oxidation}}\) |
Tertiary alcohols, such as 2-methyl-2-propanol (see Figure \(\PageIndex{3}\)), may not be oxidized in a same manner. 2-methyl-2-propanol is classified as a tertiary alcohol since the carbon atom to which the hydroxyl group is attached is directly bonded to three other carbon atoms.
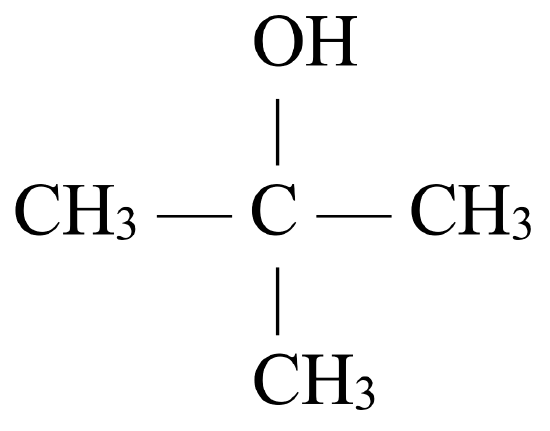 |
Nomenclature of Alcohols, Aldehydes, Ketones, and Carboxylic Acids
Alcohols, aldehydes, ketones, and carboxylic acids also bear resemblance to each other in terms of their nomenclature. Just like the alkenes and alkynes, the IUPAC nomenclature of alcohols, aldehydes, carboxylic acids, and ketones is an extension of the guidelines used to name the alkanes. While the rules used to name branched oxygen-containing organic molecules have the potential to be complex, this text only examines the nomenclature of straight-chain alcohols, aldehydes, carboxylic acids, and ketones.
Here are the basic guidelines:
☞ IUPAC Nomenclature of Straight-Chain Alcohols, Aldehydes, Carboxylic Acids, and Ketones
Alcohols, aldehydes, carboxylic acids, and ketones are named using the following convention:
- Count the number of carbon atoms in the parent chain and write the name of the alkane that has the same number of carbon atoms.
- Drop –e from the alkane name.
- If the compound is a(n):
- alcohol, replace with the suffix –ol.
- The position of the hydroxyl group is assigned a number by counting in the direction that gives the lowest number if the alcohol has three or more carbon atoms in the parent chain.
- aldehyde, replace with the suffix –al.
- carboxylic acid, replace with a suffix of –oic acid (Note: there is a space in the name).
- ketone, replace with –one.
- The position of the carbonyl group is numbered is assigned a number by counting in the direction that gives the lowest number.
- alcohol, replace with the suffix –ol.
✅ Example \(\PageIndex{1}\)
Write the IUPAC name for each molecule.

- CH3CH2OH
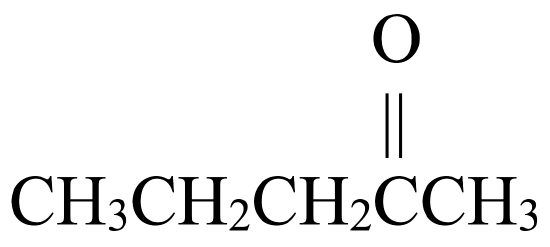
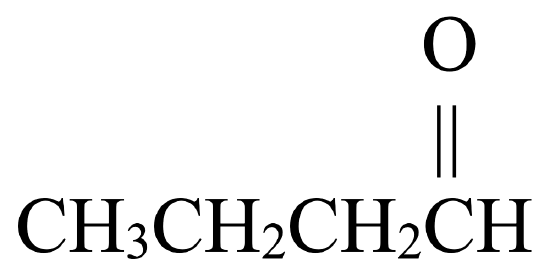
Solution
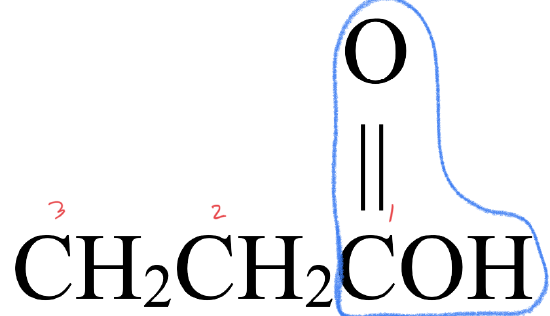
- This molecule has an alkyl group that is bonded to a carboxyl group,
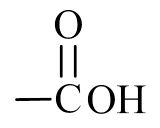 . This gives it a structure that fits a carboxylic acid,
. This gives it a structure that fits a carboxylic acid, 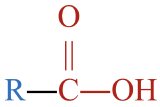 .
. - The parent chain has three carbon atoms. An alkane with three carbons is called propane.
- To name the carboxylic acid, drop –e from propane and replace with the suffix –oic acid to give propanoic acid.
- This molecule has an alkyl group that is bonded to a carboxyl group,

- This molecule has an alkyl group attached to a hydroxyl group,
 . This gives it a structure that fits an alcohol,
. This gives it a structure that fits an alcohol,  .
. - The parent chain has two carbon atoms. An alkane with two carbons is called ethane.
- To name the alcohol, drop –e from ethane and replace with the suffix –ol to give ethanol.
- There are less than three C atoms in the alcohol, so the position of the hydroxyl group is not assigned a number.
- This molecule has an alkyl group attached to a hydroxyl group,
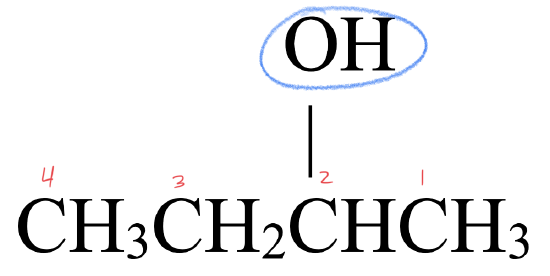
- This molecule has an alkyl group attached to a hydroxyl group,
 . This gives it a structure that fits an alcohol,
. This gives it a structure that fits an alcohol,  .
. - The parent chain has four carbon atoms. An alkane with four carbons is called butane.
- To name the alcohol, drop –e from butane and replace with the suffix –ol to give butanol.
- The hydroxyl group is attached to the 2nd carbon atom. The full name is 2-butanol.
- This molecule has an alkyl group attached to a hydroxyl group,
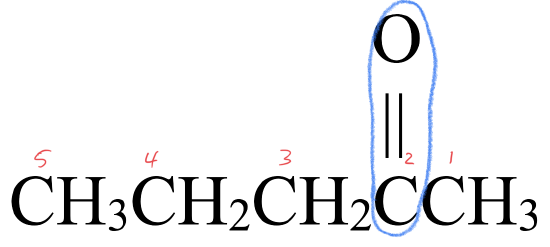
- This molecule has an alkyl group attached to each side of a carbonyl group,
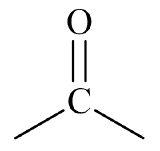 . This gives it a structure that fits a ketone,
. This gives it a structure that fits a ketone,  .
. - The parent chain has five carbon atoms. An alkane with three carbons is called pentane.
- To name the ketone, drop –e from pentane and replace with the suffix –one to give pentanone.
- The carbonyl group is located at the 2nd carbon atom. The full name is 2-pentanone.
- This molecule has an alkyl group attached to each side of a carbonyl group,
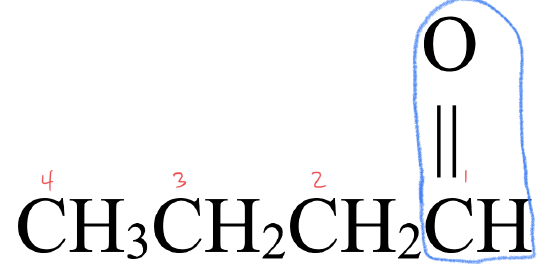
- This molecule has a carbonyl group,
 , located between an alkyl group and an H atom. This gives it a structure that fits an aldehyde,
, located between an alkyl group and an H atom. This gives it a structure that fits an aldehyde, 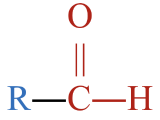 .
. - The parent chain has four carbon atoms. An alkane with four carbons is called butane.
- To name the aldehyde, drop –e from butane and replace with the suffix –al to give butanal.
- This molecule has a carbonyl group,
✏️ Exercise \(\PageIndex{1}\)
Write the IUPAC name for each molecule.
- CH3CH2CH2CH2OH


- Answer A
- 1-butanol
- Answer B
- hexanal
- Answer C
- ethanoic acid
- Answer D
- 3-pentanone
✅ Example \(\PageIndex{2}\)
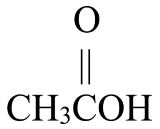
Write the condensed structural formula for each.
- 2-propanone
- pentanal
Solution
Begin by drawing the skeleton structure. The prefix prop– indicates the parent chain has three carbon atoms. A suffix of –one indicates this is a ketone, so it has a carbonyl group,  . The 2 indicates that the carbonyl group is located at the 2nd carbon atom in the parent chain.
. The 2 indicates that the carbonyl group is located at the 2nd carbon atom in the parent chain.
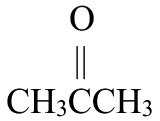
From here, fill in the H atoms. Each hydrogen makes one bond, while carbon makes four. This structure fits a ketone, 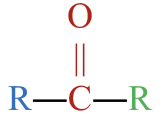 .
.
Begin by drawing the skeleton structure. The prefix pent– indicates the parent chain has five carbon atoms. A suffix of –al indicates this is an aldehyde, so it has a carbonyl group,  , located at the first carbon atom.
, located at the first carbon atom.
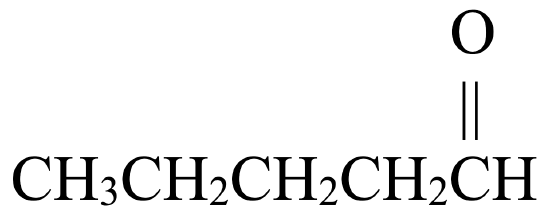
From here, fill in the H atoms. Each hydrogen makes one bond, while carbon makes four. This structure fits an aldehyde,  .
.
✏️ Exercise \(\PageIndex{2}\)
Write the condensed structural formula for each.
- butanoic acid
- methanol
- Answer A
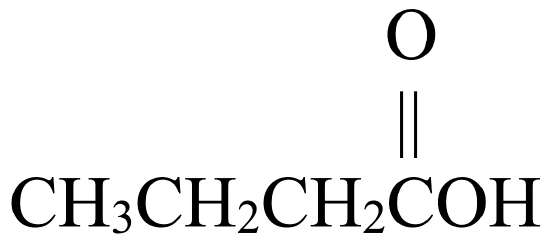
- Answer B
- CH3OH
✏️ Exercise \(\PageIndex{3}\)
Why are numbers not assigned to the functional group in either aldehydes or carboxylic acids?
- Answer
- A number is not assigned to the position of the carbonyl group in an aldehyde, since it is always located at the first carbon atom. If the carbonyl group is somewhere in the middle of the molecule, the compound would be classified as a ketone. A number is not assigned to the position of the carboxyl group in a carboxylic acid, since it is always located at the first carbon atom.
Summary
- Alcohols can be classified as primary, secondary, or tertiary based on the number of carbon atoms bonded to the carbon containing the hydroxyl group.
- Primary alcohols may be oxidized to aldehydes and carboxylic acids. Secondary alcohols may be oxidized to ketones.
- Nomenclature rules for straight-chain alcohols, aldehydes, carboxylic acids, and ketones are given.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.