12.8: Esters
- Page ID
- 355095
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Perfumes contain ingredients from a number of sources. Musk is obtained from animals, but the vast majority of perfume components are obtained from plants. Approximately 2,000 plant species have been used as sources for perfume materials. The needed chemicals are extracted using solvent extraction or distillation. The oils are diluted with ethanol to varying degrees, depending on the price of the finished product – the less ethanol present (meaning there is a higher percentage of the active scent ingredients), the more expensive the perfume.
Esters
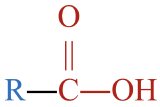
An ester is an organic compound that is a derivative of a carboxylic acid. While esters occur naturally, they are easily synthesized by reacting an alcohol (shown in red) with a carboxylic acid (shown in blue):
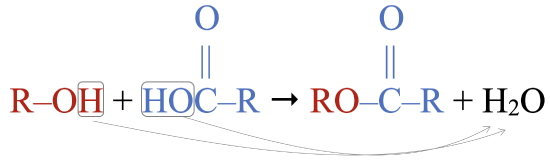
Note that the structure of the carboxylic acid was flipped 180º to show the carboxyl group on the left, so it is easier to see how the portions of the ester (shown to the right of the arrow in red and blue) derived from the alcohol and the carboxylic acid fit together. The alcohol sheds an H atom, while the carboxylic acids sheds an –OH group. The H and –OH come together to form water, shown as the other product.
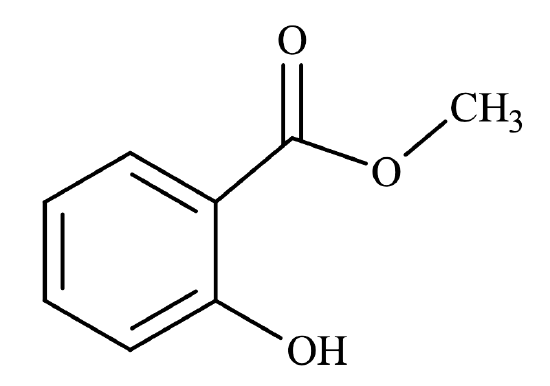
One of the cool things about the reaction to form an ester is that the progress of the reaction may be followed by the sense of smell, rather than visually. Esters tend to have pleasant odors and are very commonly found in plants – they are responsible for many distinct odors and flavors. For example, methyl salicylate has the odor and flavor of oil of wintergreen, while propyl ethanoate has that of a pear (see Figure \(\PageIndex{1}\)).
 |
 |
 |
 |
On the other hand, carboxylic acids tend to have strong, foul odors. Hexanoic acid has a common name of caproic acid, which comes from the Latin word caper, which means goat. In other words, hexanoic acid has the scent of billy goats. The smell associated with blue cheese or parmesan cheese comes from butanoic acid, which has a common name of butyric acid, and was named from the smell of rancid butter.
Just like an ester may be chemically derived from an alcohol and carboxylic acid, so may its name. Here are the basic guidelines:
☞ IUPAC Nomenclature of Esters
Esters are named using the following convention:
- The portion of the ester derived from the alcohol is named as an alkyl group.
- Note: the number 1 is dropped from the name of an alkyl group.
- Example: butyl group (the alkyl group derived from 1-butanol)
- The portion derived from the carboxylic acid named by
- Dropping –ic acid from the acid name and replacing with a suffix of –ate.
- Example: propyl butanoate (derived from 1-propanol and butanoic acid)
✅ Example \(\PageIndex{1}\)
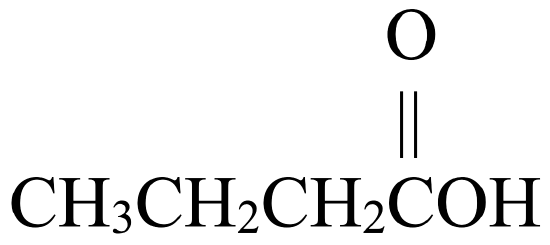
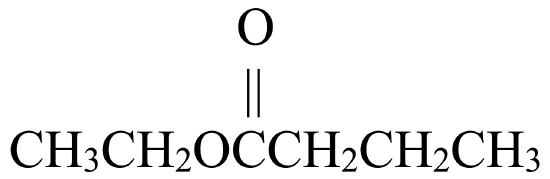
Write the IUPAC name for each molecule shown in the reaction below:
 +
+  →
→ 
Solution
- The first molecule is an alcohol,
 , that has two carbon atoms. This gives it the name ethanol.
, that has two carbon atoms. This gives it the name ethanol. - The second molecule is a carboxylic acid,
 , that has four carbon atoms. This gives it the name butanoic acid.
, that has four carbon atoms. This gives it the name butanoic acid. - The third molecule is an ester,
 .
.
- The portion derived from the alcohol has two carbon atoms and is named as an alkyl group, or ethyl.
- The portion derived from the carboxylic acid has four carbon atoms. It is named by dropping –ic acid from butanoic acid and replacing with a suffix of –ate to give butanoate.
- Ultimately, this allows us to arrive at a name of ethyl butanoate for the ester.
✏️ Exercise \(\PageIndex{1}\)
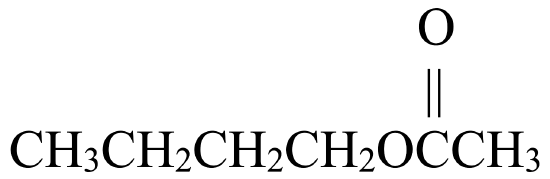
Write the IUPAC name for each molecule shown in the reaction below, from left to right:
 +
+ 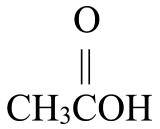 →
→ 
- Answer
- 1-butanol, ethanoic acid, butyl ethanoate
Summary
- An ester is an organic compound that is a derivative of a carboxylic acid, in which the hydrogen atom of the hydroxyl group has been replaced with an alkyl group.
- Nomenclature rules for straight-chain esters are given.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.



