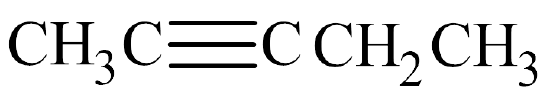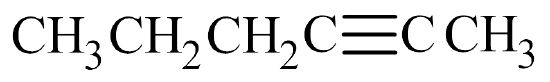12.5: Alkenes and Alkynes
- Page ID
- 355089
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
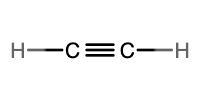
One of the most effective ways to cut metal is with an oxy-acetylene torch. Very high temperatures are obtained when acetylene burns in oxygen. Mixed 1:1 with oxygen, a temperature of over 3000°C may be achieved. The amount of energy released is high – the net heat of combustion is 1300 kJ/mol. Safety precautions need to be observed, as the gas is very explosive. The oxy-acetylene torch is one of the top tools for welding and cutting. Acetylene is also known as ethyne.
Alkenes and Alkynes
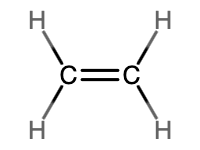
Alkenes and alkynes are two different classes of unsaturated hydrocarbons. An alkene has one or more carbon-carbon double bonds, while an alkyne has one or more carbon-carbon triple bonds. The fewest carbon atoms possible in either an alkene or alkyne is two. Whenever an additional bond is placed between two carbon atoms, it displaces two hydrogen atoms. Since alkanes have a general formula of CnH2n+2, this means that alkenes have a general formula of CnH2n, and alkynes have a general formula of CnH2n–2. Table \(\PageIndex{1}\) provides a side-by-side comparison of alkenes and alkynes.
Cis-Trans Isomerism
Isomers were previously defined as substances that have the same molecular formula, but a different arrangement of atoms. Many alkenes form a second type of isomer called a geometric isomer. In a set of geometric isomers, the same types of atoms are attached to each other in an identical order, but the geometries of the two molecules differ.
Geometric isomers in hydrocarbons are possible when the carbon atoms involved in a carbon-to-carbon double bond are located in any position other than an end/terminal carbon atom. In other words, an alkene would need at least four carbon atoms for geometric isomers to be possible. Geometric isomers of alkenes differ in the orientation of the groups on either side of a carbon-to-carbon double bond. Unlike a single bond, where atoms are free to rotate along the axis between two atoms, a double bond prevents rotation along the axis. As a result, the atoms bonded to the double-donded carbon atoms are held in fixed positions.
The two geometric isomers of 2-butene, \({\mathrm{CH}}_3\mathrm{CH}={\mathrm{CHCH}}_3\), are shown in Table \(\PageIndex{2}\) below. Notice that in the cis– isomer, the chain builds in each direction on the same side of the double bond, while in the trans– isomer, the chain builds in each direction on the opposite sides of the double bond.
| cis-2-butene | trans-2-butene | |
|---|---|---|
| Ball-and-Stick Model | ||
| Condensed Structural Formula | 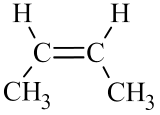 |
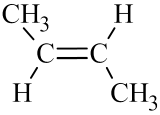 |
| Line formula |  |
 |
IUPAC Nomenclature of Alkenes and Alkynes
The IUPAC nomenclature of alkenes and alkynes is an extension of the guidelines used to name the alkanes. While the rules used to name branched alkenes and alkynes have the potential to be complex, this text only examines the nomenclature of straight-chain alkenes and alkynes.
Here are some basic guidelines:
☞ IUPAC Nomenclature of Straight-Chain Alkenes and Alkynes
- Count the number of carbon atoms in the parent chain and write the name of the alkane that has the same number of carbon atoms.
- For an alkene, drop –ane from the alkane name and replace with the suffix –ene.
- For an alkyne, drop –ane from the alkane name and replace with the suffix –yne.
- Locate and assign a number to the slot position of the carbon-to-carbon double or triple bond.
- Count in the direction that gives the lowest number.
- Place the number in front of the entire name, separating the number and name with a hyphen (Example: 2-pentyne).
- For alkenes: If the double bond is in any slot position other than the first, you must tell whether the alkene is a cis– or trans– isomer.
- If the chain builds in each direction on the same side of the double bond, it is a cis– isomer.
- If the chain builds in each direction across the double bond, it is a trans– isomer.
- The prefix cis– or trans– is written before the slot position (Example: trans-2-pentene).
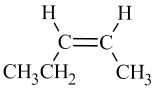
✅ Example \(\PageIndex{1}\)
Write the IUPAC name for each molecule.
Solution
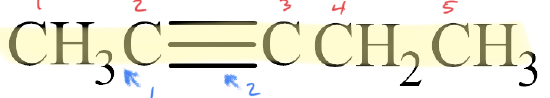
- The parent chain has five carbon atoms, so the prefix is pent–. A triple bond makes this an alkyne, so the suffix is –yne. Putting the two together gives us pentyne.
- The triple bond is located in the second slot. Therefore, this is 2-pentyne.
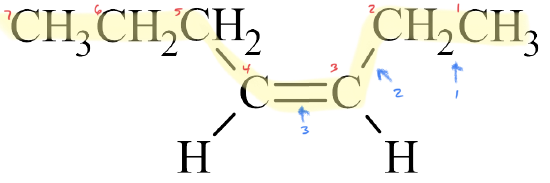
- The parent chain has seven carbon atoms, so the prefix is hept–. A double bond makes this an alkene, so the suffix is –ene. Putting the two together gives us heptene.
- The double bond is located in the third slot. Therefore, this is 3-heptene.
- The double bond is not in the first slot, so geometric isomers are possible. Since the chain builds on the same side of the molecule (the top side, in this case), it is a cis– isomer. The full name is cis-3-heptene.
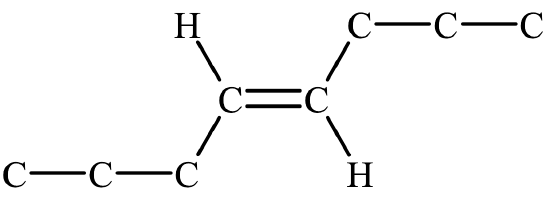
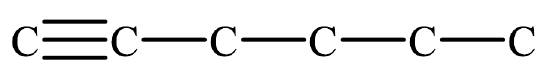
✏️ Exercise \(\PageIndex{1}\)
Write the IUPAC name for each molecule.
- Answer A
- cis-2-pentene
- Answer B
- 2-hexyne
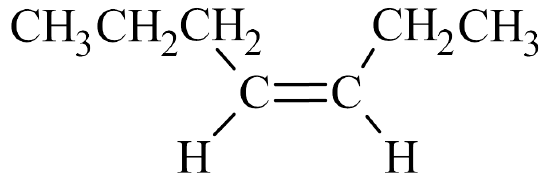
✅ Example \(\PageIndex{2}\)
Write the condensed structural formula for each.
- trans-4-octene
- 1-hexyne
Solution
Begin by drawing the skeleton structure. The prefix oct– indicates the parent chain has eight carbon atoms. A suffix of –ene indicates this is an alkene, so it has a carbon-to-carbon double bond. The prefix trans– means that the molecule builds across the double bond. The number 4 indicates the double bond is in the fourth slot.
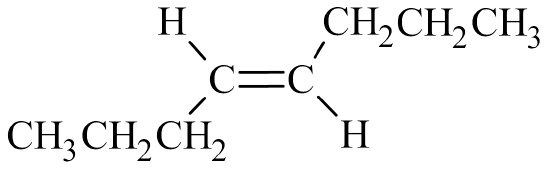
From here, fill in the H atoms. Each hydrogen makes one bond, while carbon makes four. Once all of the hydrogen atoms have been added, you can check to see if the structure conforms to the general formula for alkenes, CnH2n. Since there are 8 carbon atoms, there should be 2(8) = 16 hydrogen atoms. Summing together all of the hydrogen atoms in the condensed structural formula shows the formula is indeed C8H16.
Begin by drawing the skeleton structure. The prefix hex– indicates the parent chain has six carbon atoms. A suffix of –yne indicates this is an alkyne, so it has a carbon-to-carbon triple bond. The number 1 indicates the triple bond is in the first slot.
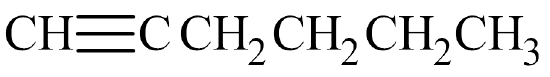
From here, fill in the H atoms. Each hydrogen makes one bond, while carbon makes four. Once all of the hydrogen atoms have been added, you can check to see if the structure conforms to the general formula for alkynes, CnH2n–2. Since there are 6 carbon atoms, there should be 2(6) – 2 = 10 hydrogen atoms. Summing together all of the hydrogen atoms in the condensed structural formula shows the formula is indeed C6H10.
✏️ Exercise \(\PageIndex{2}\)
Write the condensed structural formula for each.
- 2-butyne
- cis-2-hexene
- Answer A

- Answer B

Summary
- An alkene is a hydrocarbon with one or more carbon-to-carbon double covalent bonds.
- An alkyne is a hydrocarbon with one or more carbon-to-carbon triple covalent bonds.
- Geometric isomers are molecules with identical bonding arrangements but different geometries.
- Nomenclature rules for straight-chain alkenes and alkynes are given.
This page is shared under CK-12 and CC BY-NC-SA 4.0 licenses and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College). Original sources: https://www.ck12.org/c/chemistry/ and https://openstax.org/details/books/chemistry-2e.