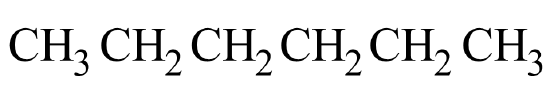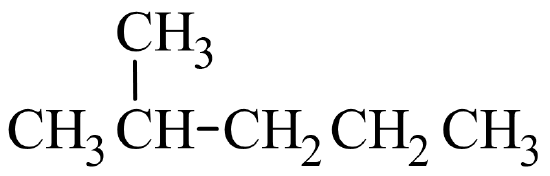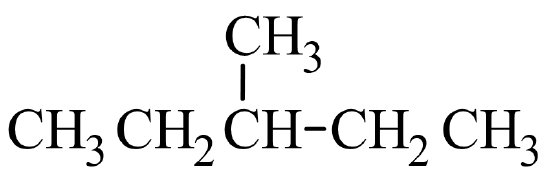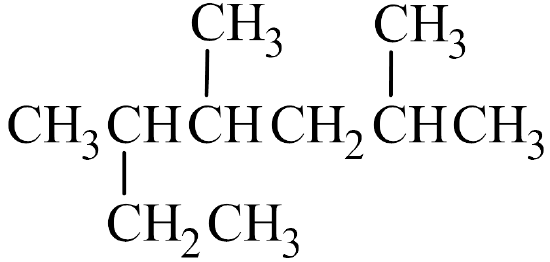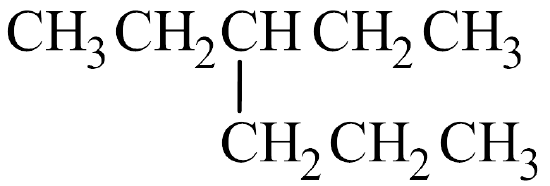12.4: Branched Alkanes
- Page ID
- 355088
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The ball-and-stick models you have seen thus far in this text use a rendering tool for viewing molecules online called JSmol. JSmol was designed to replicate molecular model kits (see Figure \(\PageIndex{1}\)) that have long been used in chemistry classes to represent three-dimensional structures of molecules.
 |
 |
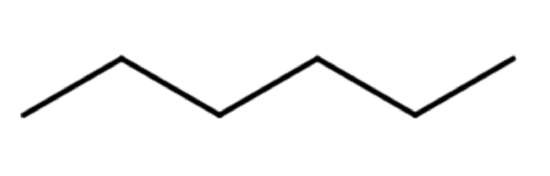
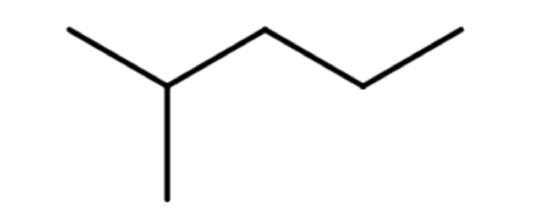
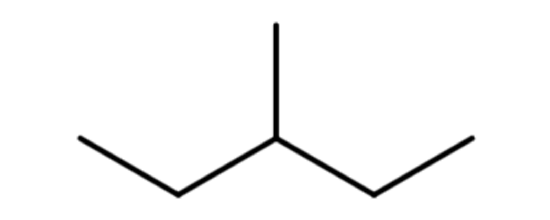
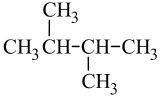
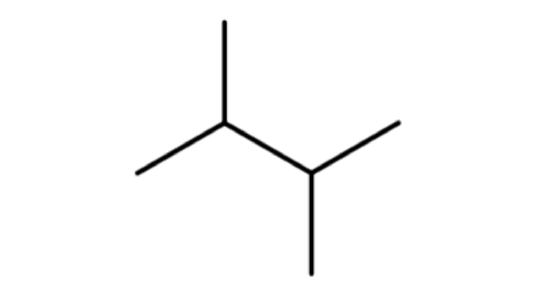
A common hands-on activity is to provide students with molecular model kits to build models that have a specified chemical formula and to compare the models. There are millions of examples that may be selected for comparison, but if that formula was C6H14, students would soon notice that there are five different ways to build a molecule of C6H14. Substances that have the same molecular formula, but a different arrangement of atoms are called isomers. The five isomers of C6H14 are shown in Figure \(\PageIndex{2}\):
Molecules rendered in JSmol may be rotated in all directions to examine molecules in different orientations. Simply "grab" onto the molecule and drag in the desired direction(s) as shown in Figure \(\PageIndex{3}\). Try rotating the molecules in Figure \(\PageIndex{2}\) above to make all 14 hydrogen atoms visible in each isomer. Go ahead...try it!
IUPAC Nomenclature
While molecules are written or drawn in two dimensions, it is important to remember that molecules are three-dimensional. Since isomers of each other are unique, they have different chemical and physical properties. The differences are sometimes small and at other times, quite significant. To distinguish isomers from one another, it is important that they be assigned a unique name. The International Union of Pure and Applied Chemistry (IUPAC) has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures.
Success with organic nomenclature begins with the ability to count carbon atoms. Similar to how Greek prefixes were used to designate a specific number of atoms in binary molecular compounds, IUPAC nomenclature uses prefixes based on the alkanes to designate a specific number of carbon atoms. Notice that Greek prefixes are used for specifying five or more carbon atoms.
Branched Alkanes
The isomers of C6H14 shown in Figure \(\PageIndex{2}\) above are shown again in Table \(\PageIndex{2}\) below along with their condensed structural formulas, line formulas, and IUPAC names. While we already learned how to name hexane and other straight-chained alkanes in the previous section, the remaining isomers of C6H14 are branched. How are the names for these branched molecules determined? Here are some basic guidelines:
☞ IUPAC Nomenclature of Branched Alkanes
- Identify and name the parent chain.
- The parent chain is the longest consecutive chain of carbon atoms in the molecule.
- The parent chain is the longest consecutive chain of carbon atoms in the molecule.
- Identify and name all branches off the parent chain as alkyl groups.
- An alkyl group is an alkane that has had one H atom removed.
- If the H atom is removed from the end carbon of a straight-chain alkane, the alkyl group is named by changing the –ane suffix of the alkane to –yl.
- The name(s) of the alkyl group(s) precedes the name of the parent chain.
- Common alkyl groups:
methyl \({\mathrm{CH}}_3\,—\) ethyl \({\mathrm{CH}}_3{\mathrm{CH}}_2\,—\) propyl \({\mathrm{CH}}_3{\mathrm{CH}}_2{\mathrm{CH}}_2\,—\) isopropyl \({\mathrm{CH}}_3\overset│{\mathrm C}{\mathrm H}_2{\mathrm{CH}}_3\) butyl \({\mathrm{CH}}_3{\mathrm{CH}}_2{\mathrm{CH}}_2{\mathrm{CH}}_2\,—\)
- Locate and assign a number to the position of each branch off the parent chain.
- For a single branch off the parent chain:
- Count in the direction that gives the lowest number.
- Place the number in front of the name of the alkyl group, separating the number and name with a hyphen (Example: 3-methyl).
- For multiple branches off the parent chain:
- Count in the direction that gives the lowest number for any branch off the parent chain.
- For branches of the same type:
- Indicate the number of that type by using the Greek prefixes di–, tri–, tetra–, etc. in front of the name of the alkyl group.
- Write the number of the position of each branch in ascending order, separating the numbers by commas.
- Example: 2,3,3-trimethylheptane
- For branches of different types:
- The alkyl groups are named in alphabetical order, with a number indicating the position of each alkyl group off the parent chain.
- Example: 3-ethyl-2-methylhexane
- For a single branch off the parent chain:
- Additional Notes:
- The name of an alkane has no spaces.
- Numbers are separated by commas. Numbers and letters are separated by hyphens.
- Whether branched or unbranched, alkanes always have a general formula of CnH2n+2.
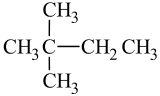
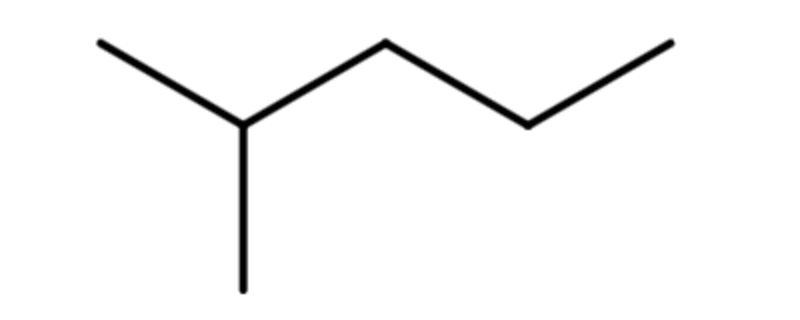
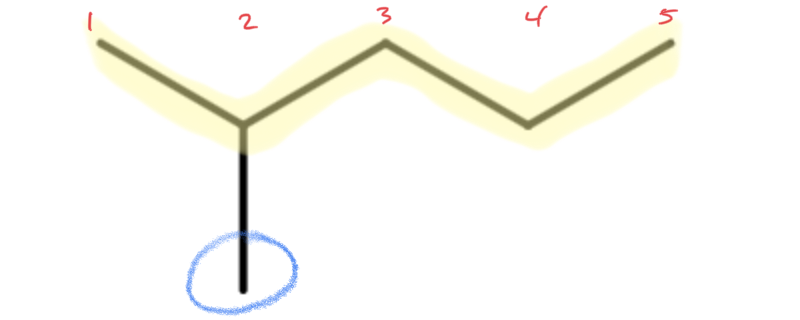
Let's look at how the name 2-methylpentane is derived. Following the guidelines for the IUPAC Nomenclature of Branched Alkanes listed above:
- The parent chain has five carbon atoms, or pentane.
- There is a one-carbon alkyl group that is branched off of the parent chain. If a one-carbon alkane is called methane, then a one-carbon alkyl group is called a methyl group. There are no spaces in the name, so methyl is written in front of pentane, giving us methylpentane.
- The methyl group is branched off of the second carbon atom in the parent chain, so 2 is written in front of methylpentane. Letters and numbers are separated with a hyphen, making it 2-methylpentane.
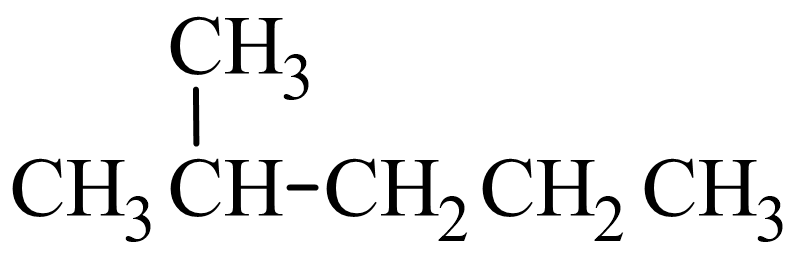 |
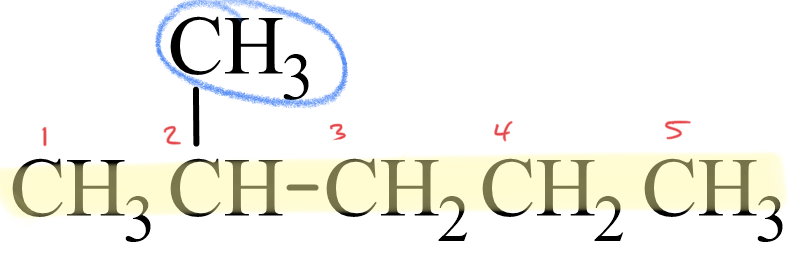 |
 |
 |
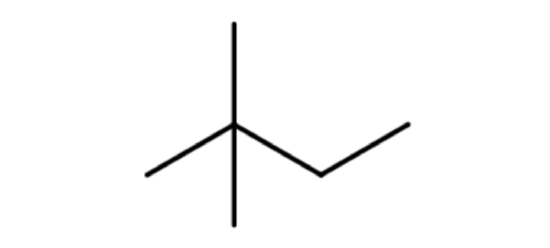
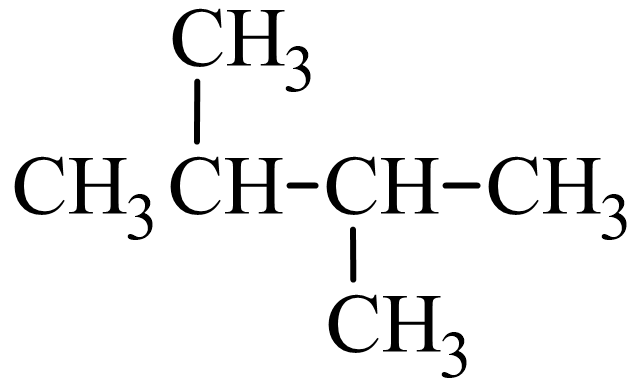
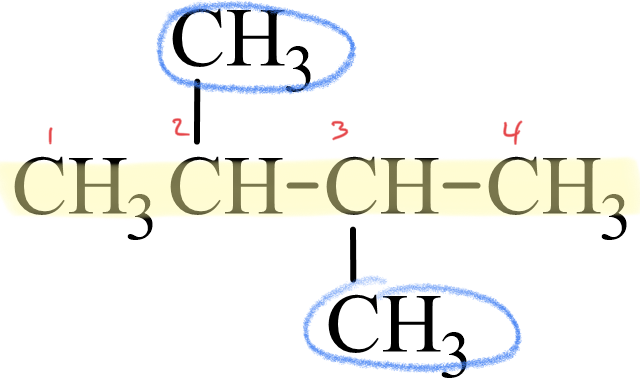
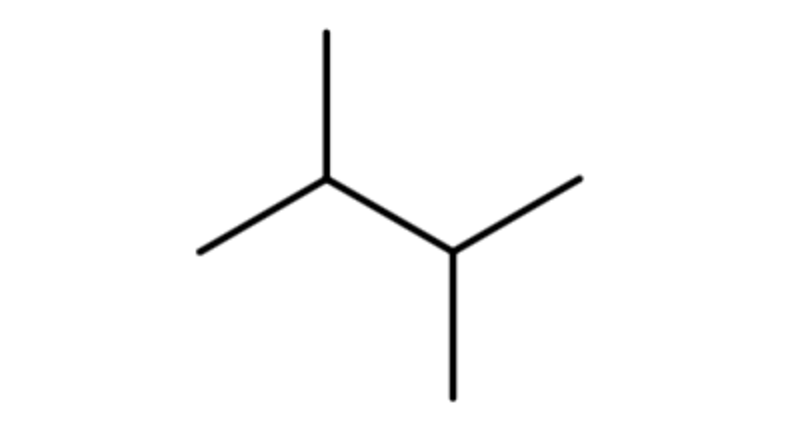
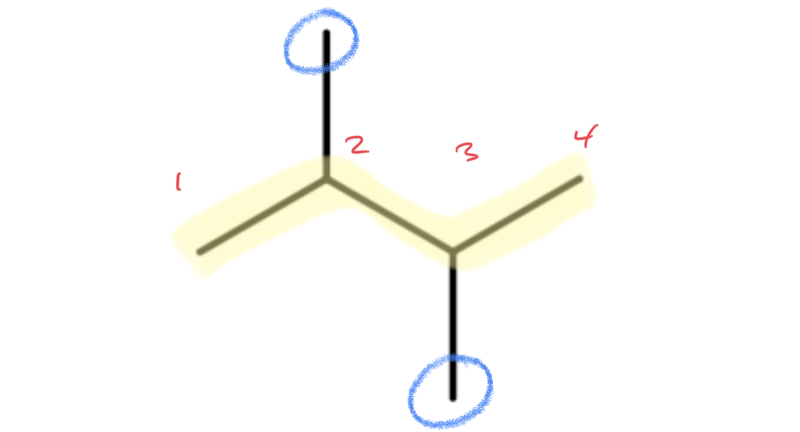
Next, lets look at how the name 2,3-dimethylbutane is derived. Following the guidelines for the IUPAC Nomenclature of Branched Alkanes listed above:
- The parent chain has four carbon atoms, or butane.
- There are two methyl groups branched off of the parent chain, which means that dimethyl is written in front of butane, making it dimethylbutane.
- One methyl group is branched off the second carbon atom of the parent chain and one is branched off the third carbon atom, so 2,3- is written in front of dimethylbutane, making it 2,3-dimethylbutane.
 |
 |
 |
 |
✅ Example \(\PageIndex{1}\)
Write the IUPAC name for each molecule.
Solution

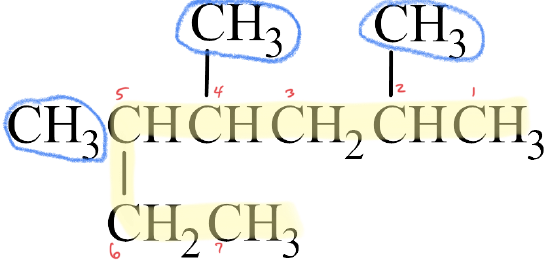
- The parent chain has seven carbon atoms, yielding a name of heptane.
- There is one ethyl group and one methyl group branched off of the parent chain.
- The parent chain is numbered from right to left, since this gives the lowest number for any branch off of the parent chain.
- The methyl group is branched off the 2nd carbon on the parent chain (making it 2-methyl) and the ethyl group is branched off the 5th carbon atom on the parent chain (making it 5-ethyl).
- Alphabetically, ethyl comes before methyl, so 5-ethyl comes first in the name, making it 5-ethyl-2-methylheptane.
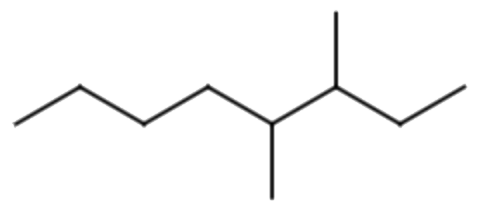
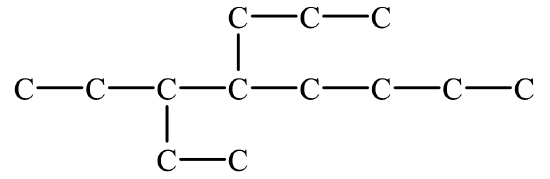
- The parent chain has seven carbon atoms, which is longer than the six consecutive carbon atoms obtained by tracing straight across the molecule. Therefore, the parent chain is named heptane.
- There are three methyl groups branched off of the parent chain, which means that trimethyl is written in front of heptane, yielding trimethylheptane.
- The parent chain is numbered from right to left, since this gives the lowest number for any branch off of the parent chain.
- The methyl groups are branched off the second, fourth, and fifth carbon atoms of the parent chain, so 2,4,5- is written in front of trimethylheptane, making it 2,4,5-trimethylheptane.
✏️ Exercise \(\PageIndex{1}\)
Write the IUPAC name for each molecule.
- Answer A
- 3,4-dimethyloctane
- Answer B
- 3-ethylhexane
✅ Example \(\PageIndex{2}\)
Write the condensed structural formula and line formula for each.
- 3-ethyl-4-propyloctane
- 3,3-dimethylhexane
Solution
It's often the easiest to begin by drawing out the skeleton structure. The name octane indicates the parent chain has eight carbon atoms. An ethyl group has two carbon atoms and is branched off the 3rd carbon atom on the parent chain. A propyl group has three carbon atoms and is branched off the 4th carbon atom on the parent chain.
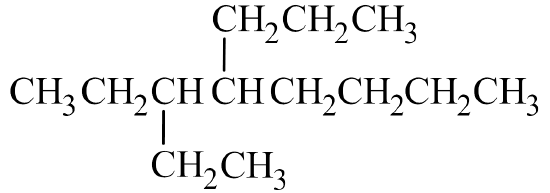
From here, fill in the H atoms. Remember that hydrogen can only make one bond, while carbon makes four. If a carbon atom is bonded to one other atom by a single bond, it can hold three hydrogen atoms. If a carbon atom makes three bonds to other atoms, it can only hold one hydrogen atom.
Once all of the hydrogen atoms have been added, you can check to see if the structure conforms to the general formula for alkanes, CnH2n+2. Since there are 13 carbon atoms, there should be 2(13) + 2 = 28 hydrogen atoms. Summing together all of the hydrogen atoms in the condensed structural formula shows the formula is indeed C13H28.
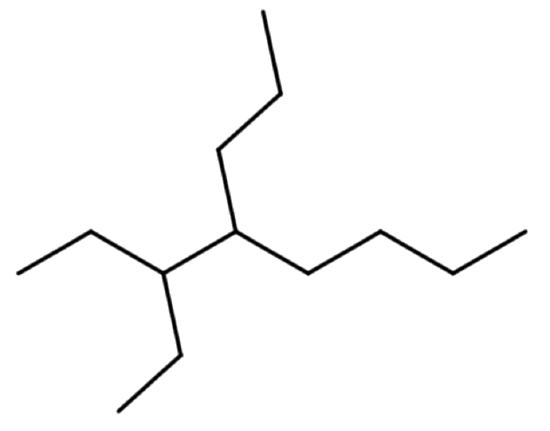
The line formula is shown above. The parent chain shows a total of eight ends or bends. There are two carbon atoms branched off the third carbon of the parent chain. There are three carbon atoms branched off the fourth carbon of the parent chain.
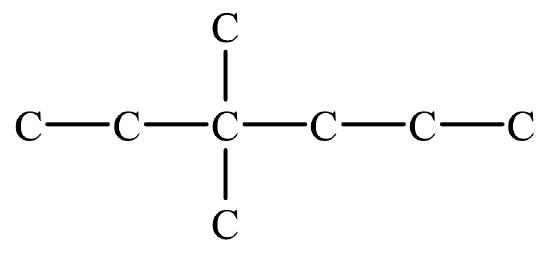
Begin by drawing the skeleton structure. The name hexane indicates the parent chain has six carbon atoms. A methyl group has one carbon atom. The prefix di– indicates there are two methyl groups. Both methyl groups are branched off the 3rd carbon atom on the parent chain.
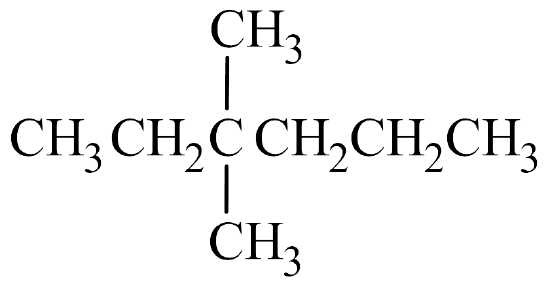
From here, fill in the H atoms. Each hydrogen makes one bond, while carbon makes four. Once all of the hydrogen atoms have been added, you can check to see if the structure conforms to the general formula for alkanes, CnH2n+2. Since there are 8 carbon atoms, there should be 2(8) + 2 = 18 hydrogen atoms. Summing together all of the hydrogen atoms in the condensed structural formula shows the formula is indeed C8H18.
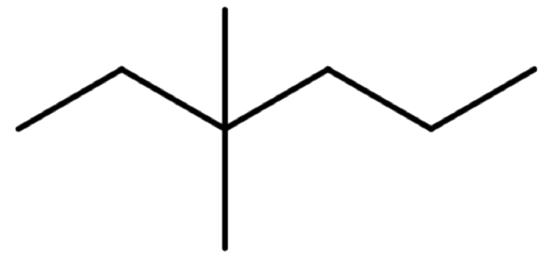
The line formula is shown above. The parent chain shows a total of six ends or bends. There are two one-carbon atom branches off the third carbon of the parent chain.
✏️ Exercise \(\PageIndex{2}\)
- Draw the line structure of 2,4-dimethylpentane.
- Write the condensed structural formula of 4-ethyl-2,3-dimethyloctane.
- Answer A
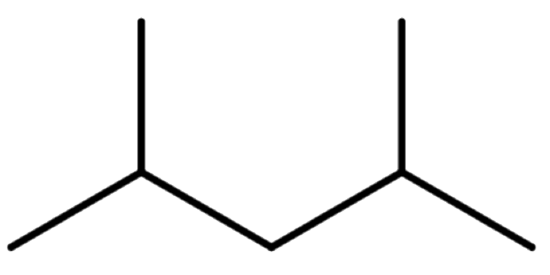
- Answer B
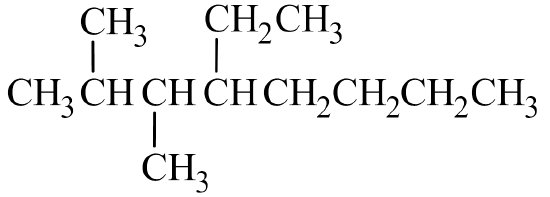
Summary
- A structural isomer is one of multiple molecules that have the same molecular formula, but different structural formulas.
- Nomenclature rules for branched hydrocarbons are given.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College). Original source: https://www.ck12.org/c/chemistry/.