11.1: Fatty Acids
- Page ID
- 434033
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To recognize the structures of common fatty acids and classify them as saturated, monounsaturated, or polyunsaturated.
Fatty acids are carboxylic acids that are structural components of fats and oils. They usually contain an even number of carbon atoms (typically 12–20), and can be classified by the presence and number of carbon-to-carbon double bonds. Note the presence of a nonpolar hydrocarbon chain (tail) and a polar carboxylic acid functional group (head) in the molecule. A fatty acid with one carbon-carbon double bond is a monounsaturated fatty acid. A fatty acid with two or more double bonds is a polyunsaturated fatty acid.

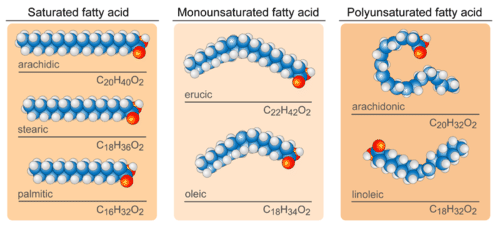
Figure \(\PageIndex{1}\): Saturated fatty acids have only single bonds while monounsaturated have one double bond and polyunsaturated have more than one double bond.
Saturated Fatty Acids
Saturated fatty acids contain all carbon-carbon single bonds. This causes the molecules to form straight chains, as shown in the figure 11.1.2. Palmitic acid is an example of a saturated fatty acid. The hydrocarbon tails of saturated fatty acids are able to be packed together very tightly with one another, interacting through London forces. London forces depends on the surface area of the molecule. The longer the hydrocarbon tail in a fatty acid, the stronger the interactions between molecules. The melting points of saturated fatty acids increases as the molecules get larger. This explains why saturated fatty acids are solids at room temperature. Animals use saturated fatty acids to store energy.
Unsaturated Fatty Acids
In an unsaturated fatty acids, the hydrocarbon chain contains at least one carbon-carbon double bond (alkene). The double bonds are usually cis. The presence of a cis double bond causes chains to bend or kink in the hydrocarbon tail (see figure 11.1.2). The bent tails in neighboring unsaturated fatty acids are farther apart from one another compared to saturated fatty acids. This reduction of contact between the tails weakens the london forces between them. So unsaturated fatty acids are liquids at room temperature. For fatty acids with the same number of carbon atoms, the presence of more double bonds lowers the melting point. Plants use unsaturated fatty acids to store energy.
Figure \(\PageIndex{2}\): Structures of saturated and unsaturated fatty acids.
Essential Fatty Acids
Two polyunsaturated fatty acids—linoleic and linolenic acids—are termed essential fatty acids because humans must obtain them from their diets. Both substances are required for normal growth and development, but the human body does not synthesize them. The average daily diet should contain about 4–6 g of the essential fatty acids.Common Fatty Acids
Table \(\PageIndex{1}\) lists some common fatty acids. Chemists often use a shorthand notation for fatty acids along with the common name because they are all carboxylic acids with different numbers of carbons and number of double bonds. This notation gives the number of carbons followed by the number of double bonds present. For example: palmitic acid is a 16-carbon saturated fatty acid, would be represented by C16:0 and palmitoleic acid is a 16-carbon monounsaturated fatty acid, would be C16:1.| Name | Number of Carbons | Number of Double Bonds | Condensed Structural Formula | Melting Point (°C) | Shorthand Notation | Source |
|---|---|---|---|---|---|---|
| lauric acid | 12 | 0 | CH3(CH2)10COOH | 44 | C12:0 | palm kernel oil |
| myristic acid | 14 | 0 | CH3(CH2)12COOH | 58 | C14:0 | oil of nutmeg |
| palmitic acid | 16 | 0 | CH3(CH2)14COOH | 63 | C16:0 | palm oil |
| palmitoleic acid | 16 | 1 | CH3(CH2)5CH=CH(CH2)7COOH | 0.5 | C16:1 | macadamia oil |
| stearic acid | 18 | 0 | CH3(CH2)16COOH | 70 | C18:0 | cocoa butter |
| oleic acid | 18 | 1 | CH3(CH2)7CH=CH(CH2)7COOH | 16 | C18:1 | olive oil |
| linoleic acid | 18 | 2 | CH3(CH2)3(CH2CH=CH)2(CH2)7COOH | −5 | C18:2 | canola oil |
| linolenic acid | 18 | 3 | CH3(CH2CH=CH)3(CH2)7COOH | −11 | C18:3 | flaxseed |
| arachidonic acid | 20 | 4 | CH3(CH2)4(CH=CHCH2)4(CH2)2COOH | −50 | C20:4 | liver |
ω-3 Fatty acids
Fats containing saturated fatty acids are considered not so healthy as they have a tendency to deposit in arteries forming plaque, i.e., atherosclerosis. It can lead to high blood pressure, rupture of arteries, or heart attack. Saturated fatty acids are more common in animal fats while unsaturated fatty acids which are considered more healthy are more common in vegetable oils.
In omega (ω) labeling the last C, i.e., the −CH3 at the end opposite to carboxyl (−COOH) group is labeled ω-1, the C next to it as ω-2, then ω-3 and so on, as as shown in the figure 11.1.3. Omega (ω) is the last alphabet in Greek, so, ω-C is the last carbon. The (ω) label is used to refer to the position of the first C=C bond from the ω-end. For example, linolenic acid in figure below is an ω-3 fatty acid.

Figure \(\PageIndex{3}\): Labelling of C′s in fatty acids: systematic IUPAC numbers are in red, nonsystematic Greek letter labels are in black, and omega- (ω-#) are in blue. (Copyright; Public domain)
It has been observed that peoples from Alaska eat a higher amount of unsaturated fats and have a low occurrence of atherosclerosis and heart attack. Fats in Alaskan food is primarily from fish, such as salmon, tuna, and herring. Vegetable oils have higher contents of ω-6 fatty acids, such as linoleic acid and arachidonic acid. Fish oil mostly contains ω-3 fatty acids, such as linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid.
 salmon
salmon tuna
tuna herring
herring
Fatty Acid Compositions of Common Fats and Oils
Table 11.1.2 shows the fatty acid compositions of some common fats and oils. The composition of any given fat or oil can vary depending on the plant or animal species it comes from as well as on dietetic and climatic factors. To cite just one example, lard from corn-fed hogs is more highly saturated than lard from peanut-fed hogs. Palmitic acid is the most abundant of the saturated fatty acids, while oleic acid is the most abundant unsaturated fatty acid. Terms such as saturated fat or unsaturated oil are often used to describe the fats or oils obtained from foods. Saturated fats contain a high proportion of saturated fatty acids, while unsaturated oils contain a high proportion of unsaturated fatty acids. The high consumption of saturated fats is a factor, along with the high consumption of cholesterol, in increased risks of heart disease.| Source | Lauric (C12:0) | Myristic (C14:0) | Palmitic (C16:0) | Stearic (C18:0) | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3) |
|---|---|---|---|---|---|---|---|
| Fats | |||||||
| butter (cow) | 3 | 11 | 27 | 12 | 29 | 2 | 1 |
| tallow | 3 | 24 | 19 | 43 | 3 | 1 | |
| lard | 2 | 26 | 14 | 44 | 10 | ||
| Oils | |||||||
| canola oil | 4 | 2 | 62 | 22 | 10 | ||
| coconut oil† | 47 | 18 | 9 | 3 | 6 | 2 | |
| corn oil | 11 | 2 | 28 | 58 | 1 | ||
| olive oil | 13 | 3 | 71 | 10 | 1 | ||
| peanut oil | 11 | 2 | 48 | 32 | |||
| soybean oil | 11 | 4 | 24 | 54 | 7 | ||
| *Totals less than 100% indicate the presence of fatty acids with fewer than 12 carbon atoms or more than 18 carbon atoms. | |||||||
| †Coconut oil is highly saturated. It contains an unusually high percentage of the low-melting C8, C10, and C12 saturated fatty acids. | |||||||