8.6: Acid and Base Strength
- Page ID
- 432731
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Describe the difference between strong and weak acids and bases.
Strong and Weak Acids
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way. Consider \(\ce{HCl(aq)}\). When \(\ce{HCl}\) is dissolved in \(\ce{H2O}\), it completely dissociates into H+(aq) and Cl−(aq) ions; all the HCl molecules become ions:
\[\ce {HCl\overset{100\%}{\rightarrow}H^{+}(aq) + Cl^{-}(aq)} \]
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid. HC2H3O2 is an example of a weak acid:
\[HC_{2}H_{3}O_{2}\overset{\sim 5\%}{\longrightarrow}H^{+}(aq)+C_{2}H_{3}O_{2}^{-}(aq)\]
Because this dissociation does not go 100% to completion, it is more appropriate to write it as a reversible reaction:
\[HC_{2}H_{3}O_{2}\rightleftharpoons H^{+}(aq)+C_{2}H_{3}O_{2}^{-}(aq)\]
As it turns out, there are very few strong acids, which are given in Table \(\PageIndex{1}\). If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid.
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid.
| Acids | Bases |
|---|---|
| HCl | LiOH |
| HBr | NaOH |
| HI | KOH |
| HNO3 | RbOH |
| H2SO4 | CsOH |
| HClO3 | |
| HClO4 |
Strong and Weak Bases
The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table \(\PageIndex{1}\)); any base not listed is a weak base. All strong bases are OH– compounds. So a base based on some other mechanism, such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base. Bases that are salts of carbonate and bicarbonate ions are also weak bases
Example \(\PageIndex{1}\): Identifying Strong and Weak Acids and Bases
Identify each acid or base as strong or weak.
- HCl
- Mg(OH)2
- C5H5N
Solution
- Because HCl is listed in Table \(\PageIndex{1}\), it is a strong acid.
- Because Mg(OH)2 is not listed in Table \(\PageIndex{1}\), it is a weak base.
- The nitrogen in C5H5N would act as a proton acceptor and therefore can be considered a base, but because it does not contain an OH compound, it cannot be considered a strong base; it is a weak base.
Exercise \(\PageIndex{1}\)
Identify each acid or base as strong or weak.
- \(\ce{RbOH}\)
- \(\ce{HNO_2}\)
- Answer a
- strong base
- Answer b
- weak acid
Chemical Equilibrium in Weak Acids and Bases
The behavior of weak acids and bases illustrates a key concept in chemistry. Does the chemical equation describing the ionization of a weak acid or base just stop when the acid or base is done ionizing? Actually, no. Rather, the reverse process, the reformation of the molecular form of the acid or base occurs, ultimately at the same rate as the ionization process. For example, the ionization of the weak acid HC2H3O2(aq) is as follows:
HC2H3O2(aq)+H2O(ℓ)→H3O+(aq)+C2H3O−(aq)
The reverse process also begins to occur:
H3O+(aq)+C2H3O−(aq)→HC2H3O2(aq)+H2O(ℓ)
Eventually, there is a balance between the two opposing processes, and no additional change occurs. The chemical reaction is better represented at this point with a double arrow:
HC2H3O2(aq)+H2O(ℓ) ⇌ H3O+(aq)+C2H3O−(aq)
The ⇌ implies that both the forward and reverse reactions are occurring, and their effects cancel each other out. The process at this point is considered to be at chemical equilibrium (or equilibrium). It is important to note that the processes do not stop. They balance out each other so that there is no further net change; that is, chemical equilibrium is a dynamic equilibrium.
Acid Ionization Constant, Ka
The ionization for a general weak acid, HA, can be written as follows:
HA(aq)⇌H+(aq)+A−(aq)
Because the acid is weak, an equilibrium expression can be written. An acid ionization constant (Ka) is the equilibrium constant for the ionization of an acid.

The acid ionization represents the fraction of the original acid that has been ionized in solution. Therefore, the numerical value of Ka is a reflection of the strength of the acid. Weak acids with relatively higher Ka values are stronger than acids with relatively lower Ka values. Because strong acids are essentially 100% ionized, the concentration of the acid in the denominator is nearly zero and the Ka value approaches infinity. For this reason, Ka values are generally reported for weak acids only.
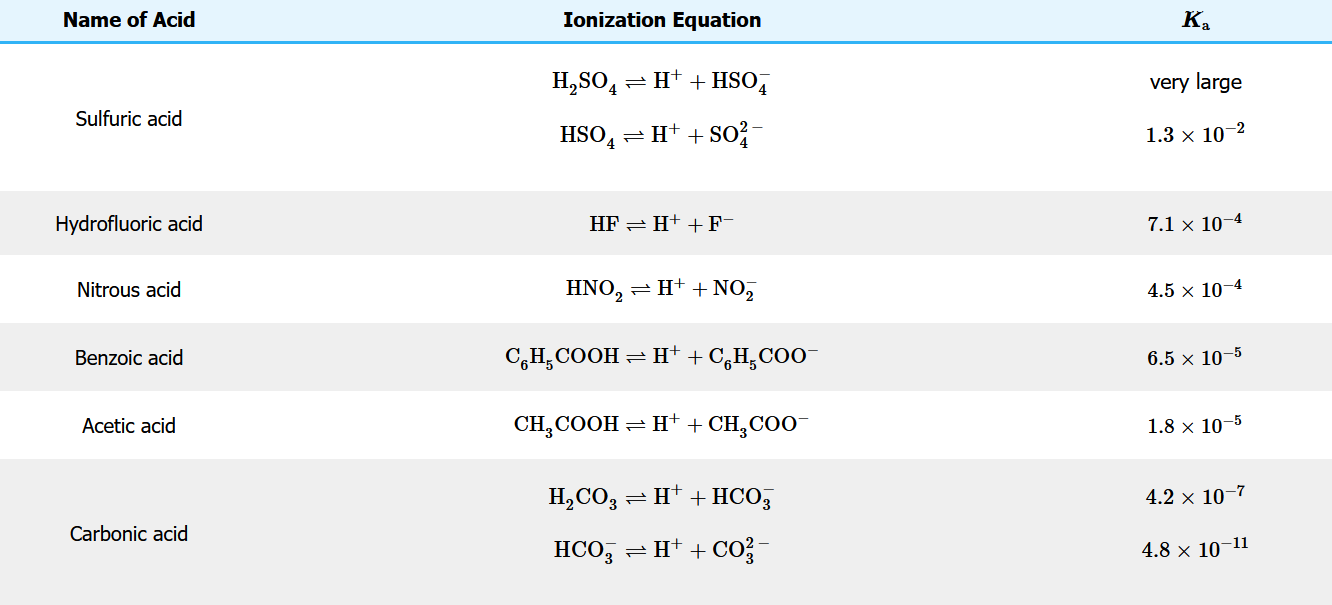
The table below is a listing of acid ionization constants for several acids. Note that polyprotic acids have a distinct ionization constant for each ionization step, with each successive ionization constant being smaller than the previous one.
Table \(\PageIndex{2}\): Acid Ionization Constants at 25oC
Key Takeaways
- Strong acids and bases are 100% ionized in aqueous solution.
- Weak acids and bases are less than 100% ionized in aqueous solution.