7.8: Fate of Pyruvate
- Page ID
- 432235
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how the presence or absence of oxygen determines what happens to the pyruvate produced in glycolysis.
Metabolism of Pyruvate
The presence or absence of oxygen determines the fates of the pyruvate produced in glycolysis. When plenty of oxygen is available (aerobic conditions), pyruvate is first converted to acetyl-CoA. However, in the absence of oxygen (that is, under anaerobic conditions), the fate of pyruvate is different in different organisms. In vertebrates, pyruvate is converted to lactate, while other organisms, such as yeast, convert pyruvate to ethanol and carbon dioxide. These possible fates of pyruvate are summarized in Figure \(\PageIndex{1}\).
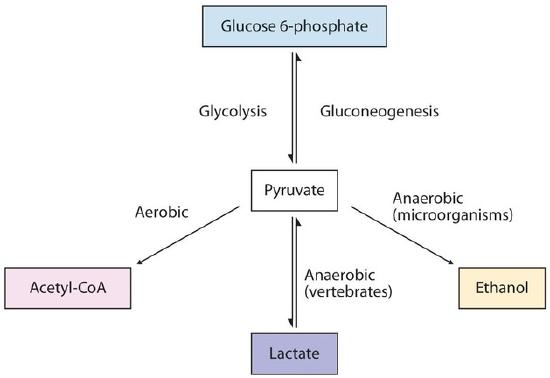
Figure \(\PageIndex{1}\): Metabolic Fates of Pyruvate
Aerobic Respiration
If oxygen is available, aerobic respiration will go forward. The pyruvate molecules produced at the end of glycolysis are transported into mitochondria. In order for pyruvate, the product of glycolysis, to enter the next pathway, it must undergo changes.
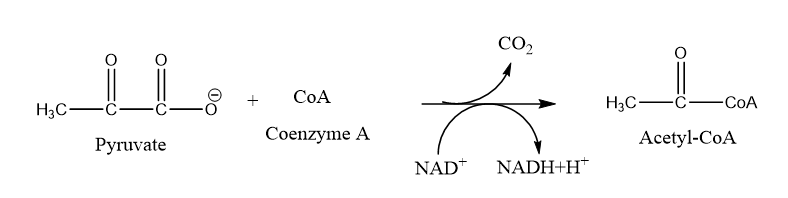
Figure \(\PageIndex{2}\): Metabolic Fates of Pyruvate During Aerobic Conditions
In the mitochondrial matrix, pyruvate will be transformed into a two-carbon compound by removing a molecule of carbon dioxide. This also produces NADH. An activating group called coenzyme A (CoA), which is made from vitamin B5 reacts with the two-carbon molecule. The resulting compound is called acetyl-CoA as shown in figure 7.8.2. Acetyl-CoA can be used in a variety of ways by the cell, but its major function is to start the citric acid cycle.
Anaerobic Respiration or Fermentation
Fermentation is the process of producing ATP in the absence of oxygen. Glycolysis breaks a glucose molecule into two pyruvate molecules, producing a net gain of two ATP and two NADH molecules. Lactic acid fermentation is the type of anaerobic respiration carried out by yogurt bacteria (Lactobacillus and others) and by your own muscle cells when you exercise.
Note: The difference between the structures of lactic acid and lactate is in the presence or absence of a H+ ion. At physiological pH the lactic acid is present as lactate ion.

Figure \(\PageIndex{3}\): Metabolic Fates of Pyruvate During Anaerobic Conditions
Lactic acid fermentation converts the 3-carbon pyruvate to the 3-carbon lactate and regenerates NAD+ in the process, allowing glycolysis to continue to make ATP in low-oxygen conditions. Since there is a limited supply of NAD+ available in any given cell, this oxidizing agent must be regenerated to allow ATP production to continue. To achieve this, NADH acts as a reducing agent, regenerating NAD+. Lactate is formed by the reduction of pyruvate as shown in Figure 7.8.3.
In the human body, the lactate ions are sent in blood to the liver, where it can be used to manufacture glucose through the process of gluconeogenesis. You may have noticed this type of fermentation in your own muscles, because muscle fatigue and pain are associated with lactate ions. Lactate ions accumulates in your muscle cells as fermentation proceeds during times of strenuous exercise. During these times, your respiratory and cardiovascular systems cannot transport oxygen to your muscle cells, especially those in your legs, fast enough to maintain aerobic respiration. To allow the continuous production of some ATP, your muscle cells use lactic acid fermentation.
For Lactobacillus bacteria, the acid resulting from fermentation kills bacterial competitors in buttermilk, yogurt, and some cottage cheese. The benefits extend to humans who enjoy these foods.

Alcoholic Fermentation in Yeast
The purpose of fermentation in yeast is the same as that in muscle and bacteria, to replenish the supply of NAD+ for glycolysis. This process occurs in two steps as shown in figure 7.8.4. It is used to make bread, wine, and biofuels.
- Alcoholic fermentation consists of pyruvate being first converted into acetaldehyde by the enzyme pyruvate decarboxylase and releasing CO2.
- In the second step acetaldehyde is reduced to ethanol using alcohol dehydrogenase and producing NAD+ in the process. It is this recycled NAD+ that can be used to continue on with glycolysis.

Figure \(\PageIndex{4}\): Metabolic Fates of Pyruvate in Yeast.
Ethanol is produced by alcoholic fermentation of the glucose in corn or other plants. This type of fermentation also explains why bread dough rises. Yeasts in bread dough use alcoholic fermentation and produce carbon dioxide gas. The gas forms bubbles in the dough, which cause the dough to expand. The bubbles also leave small holes in the bread after it bakes, making the bread light and fluffy. The small holes in bread are formed by bubbles of carbon dioxide gas. The carbon dioxide gas was produced by alcoholic fermentation carried out by yeast.

Summary
- In the presence of oxygen, pyruvate is converted to acetyl-CoA which then enters the citric acid cycle to produce more ATP.
- In the absence of oxygen, pyruvate is converted to lactate, and NADH is re-oxidized to NAD+.
- In alcoholic fermentation, pyruvic acid changes to alcohol and carbon dioxide. This type of fermentation is carried out by yeasts and some bacteria.


