4.4: Shapes of Molecules
- Page ID
- 430690
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Predict the general shape of a simple covalent compounds.
Molecular Shape
Ionic compounds have extended crystal lattices. Atoms in a molecule or polyatomic ion are arranged into geometric patterns that allow their electron pairs to get as far away from each other as possible. This minimizes the repulsive forces between them. Covalent bonds, are composed of negatively charged electrons, that tend to repel one another. This concept is called the valence shell electron pair repulsion (VSEPR) theory.
Most simple geometric structures fall into the following patterns:
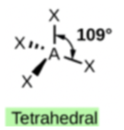
Tetrahedral
When four pairs of shared electrons for a total of 4 electron groups are around a central atom the result is a tetrahedral shape. CH4, CCl4, CHCl3, CH2Cl2 etc., all have tetrahedral geometry around the central atom.

Figure \(\PageIndex{1}\): Tetrahedral shape of CH4.
Pyramidal
When there are three pairs of shared electrons and one lone pair of unshared electrons for a total of 4 electron groups around a central atom the result is a pyramidal shape. NH3 has one lone electron pair and three bonded electron pairs. These four electron pairs repel each other and adopt a tetrahedral arrangement. However, the shape of the molecule is described in terms of the positions of the atoms, not the lone electron pairs. Thus, NH3 is said to have a pyramidal shape, not a tetrahedral one.

Figure \(\PageIndex{2}\): Pyramidal shape of NH3.
Planar
When there are three groups of shared electrons around a central atom, two of these groups are single bonds and one group is a double bond made up of two pairs of shared electrons. There are no lone pairs of electrons around the central atom.
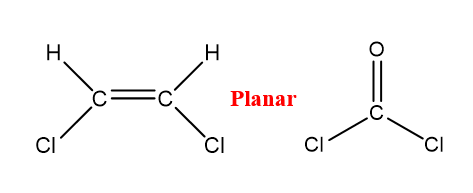
Figure \(\PageIndex{3}\): Planar shape around the central atom carbon.
The shapes of molecules with multiple bonds are determined by treating the double bond as one group. Thus, formaldehyde (CH2O) has a planar shape.

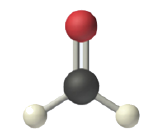
Figure \(\PageIndex{4}\): Planar shape of CH2O.
Bent
When two groups of shared electrons in single bonds and two pairs of unshared electrons are around a central atom the result is the bent shape around the central atom. An important example is the bent shape around the oxygen central atom in water (H2O).
Or
When two groups of shared electrons in a single bond and a double bond and one pair of unshared electrons are around a central atom the result is also a bent geometry around the central atom.


Figure \(\PageIndex{5}\): Bent shape of H2O and SO2.
Linear
When there are two groups of shared electrons, usually double bonds with two shared electron pairs between two atoms, and no unshared electrons around a central atom the geometry is linear.

Figure \(\PageIndex{6}\): Linear shape of CS2.
When there are two atoms in a molecule or ion, and there is no central atom (HBr for example), the geometry is also linear.
In summary, to determine the molecular geometry:
Step 1: Draw the line bond structure.
Step 2: Count the number of bonds (a double/triple bond counts as one) and lone pairs around the central atom.
Step 3: Determine the molecular geometry. The molecular geometry depends on the number of bonds and/or lone pairs around the central atom.
If a molecule has 2 bonds and 0 lone pairs, it is linear. If a molecule has 2 bonds and 1 lone pair, it is bent or angular. If a molecule has 4 bonds and 0 lone pairs, it is tetrahedral. If a molecule has 3 bonds and 1 lone pair, it is a pyramid. If a molecule has 2 bonds and 2 lone pairs, it is bent or angular.
What is the geometry of the ammonium ion, NH4+? Its Lewis structure is shown below. How is this different from ammonia, NH3?
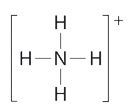
Solution
In ammonium ion, the central atom N has 4 bonds and no lone pair. Hence, this is tetrahedral.
In ammonia (NH3), shown below, N has 3 bonds and one lone pair.
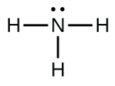
Hence, the shape of this molecule is pyramidal.

Exercise \(\PageIndex{1}\)
What is the molecular shape of nitrosyl chloride, a highly corrosive, reddish-orange gas? Its Lewis structure is shown below.
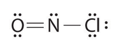
- Answer
-
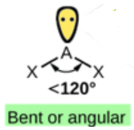
Focus on the central atom, N. It has a double bond to O, count this as one bond. It also has a single bond to Cl. Thus, N has 2 bonds and one lone pair. These 3 electron pairs will spread out 120 degrees from each other. But, since the shape is defined by the arrangement of the atoms only, the shape is bent or angular.
Biochemistry Link: Prions
The shape that a molecule takes can influence its biological function. Prions (proteinaceous infection particles) are a class of proteins that have been identified as the cause of mad cow disease in cattle and scrapie in sheep. Protein are large macromolecules made of amino acids. They have a favored shape and fold into a compact structure. In prion diseases the normal prion PrPc is twisted into an infectious abnormal shape PrPsc. PrPsc induces PrPc to change shape and become PrPsc. As PrPsc accumulates in animals, sponge like holes form in the brain causing dizziness, seizures, and death. Prion disease is infectious and can be transferred from one species to another. Infections in humans are linked to eating mad cow infected beef.

Figure \(\PageIndex{7}\): Models of PrPc (left) and PrPsc(right)
Key Takeaways
- Simple molecules have geometries around a central atom.


