Temperature Effects on Solubility
- Page ID
- 23408
The solubility of solutes is dependent on temperature. When a solid dissolves in a liquid, a change in the physical state of the solid analogous to melting takes place. Heat is required to break the bonds holding the molecules in the solid together. At the same time, heat is given off during the formation of new solute -- solvent bonds.
- CASE I: Decrease in solubility with temperature: If the heat given off in the dissolving process is greater than the heat required to break apart the solid, the net dissolving reaction is exothermic (energy given off). The addition of more heat (increases temperature) inhibits the dissolving reaction since excess heat is already being produced by the reaction. This situation is not very common where an increase in temperature produces a decrease in solubility.
- CASE II: Increase in solubility with temperature: If the heat given off in the dissolving reaction is less than the heat required to break apart the solid, the net dissolving reaction is endothermic (energy required). The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids.
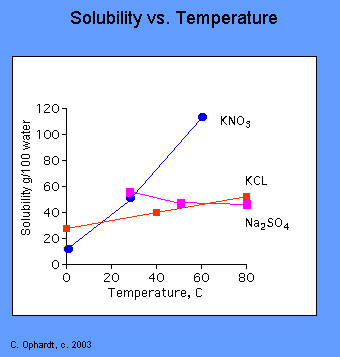
Figure: Temperature dependent solubilities of three salts in water.
The use of first-aid instant cold packs is an application of this solubility principle. A salt such as ammonium nitrate is dissolved in water after a sharp blow breaks the containers for each.
\[NH_4NO_{3(s)} \rightarrow NH_{4(aq)}^+ + NO^-_{3(aq)}\]
The dissolving reaction is endothermic and requires heat. Therefore the heat is drawn from the surroundings and the pack feels cold.
Contributors and Attributions
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook

