5: Thermodynamic Cycles
- Page ID
- 413931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The first law of thermodynamics places no restrictions on the conversion of energy from one form to another. For example, let’s consider once again the Joule experiment (Figure 3.1.1). If we design a cycle that goes from the gas on the left chamber only to the gas equilibrated in both chambers and backward, as in Figure \(\PageIndex{1}\), there are no restrictions imposed on this hypothetical cycle by the first law.
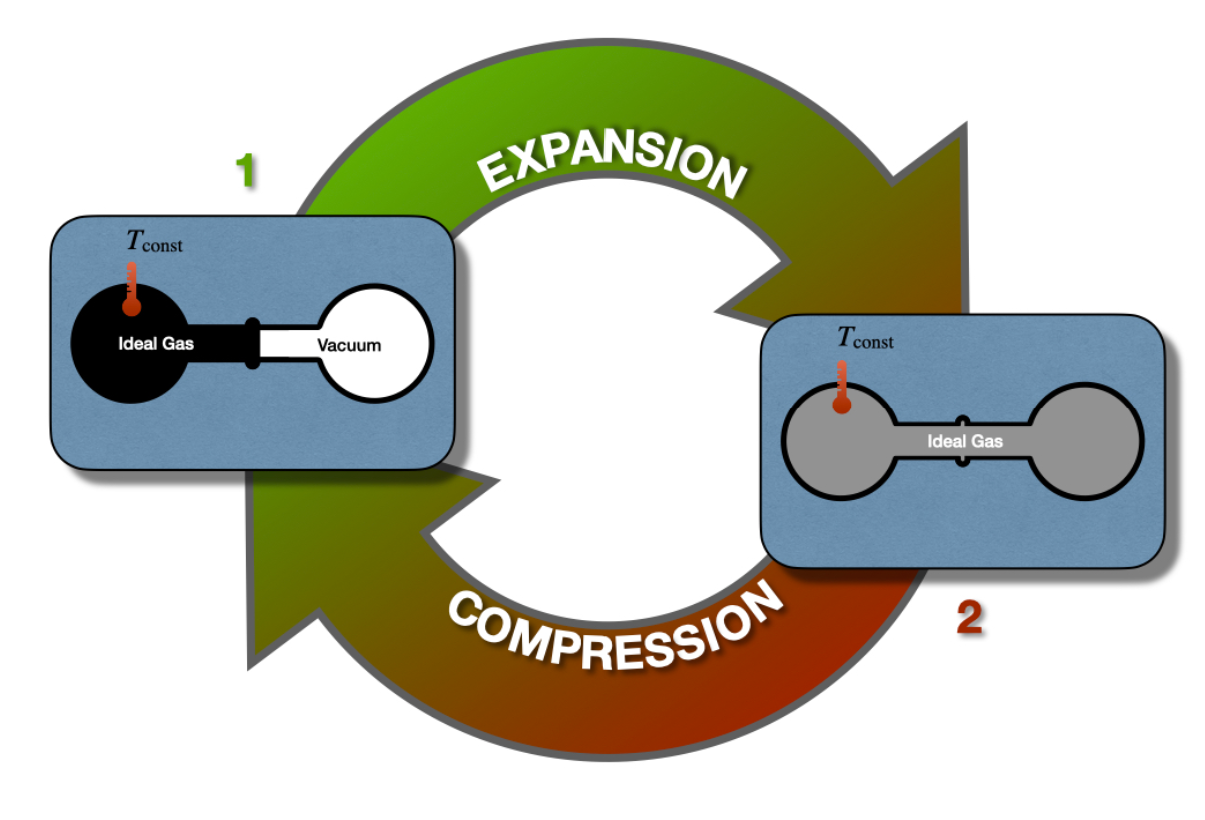
As we saw in section 3.1.1, states 1 and 2 have exactly the same energy at constant temperature. Restricting the analysis to the information contained in the first law, the ideal gas could hypothetically go from state 1 (all gas in the left chamber) to state 2 (gas in both chambers), as well as spontaneously close the cycle back from state 2 to state 1, without external intervention. While the transformation from 1 \(\rightarrow\) 2 is intuitively spontaneous (it’s the same transformation that we considered in section 3.1.1), the backward transformation from 2 \(\rightarrow\) 1 is clearly not as intuitive. In this case, the gas should spontaneously compress back to the left side, leaving a vacuum on the right chambers, without interventions from the outside. This transformation is clearly never observed. A gas just does not spontaneously concentrate on one side of a room, leaving a vacuum on the other side. In fact, when we need to create a vacuum, a lot of energy must be spent. Suppose we use exclusively information contained in the first law. In this case, there is nothing that might suggest a system’s preference to perform the transformation 1 \(\rightarrow\) 2, while restricting the 2 \(\rightarrow\) 1 from happening spontaneously. Both states have the same energy, and
\[ \oint dU=0, \nonumber \]
James Joule himself was indeed convinced that this must be the case and that we don’t observe the backward transformation in practice only because we cannot build ideal machines.\(^1\) Another scientist of that era was not convinced. William Thomson, the 1st Baron Kelvin (1824–1907), was unsure about this idea, and invested substantial resources to try to prove Joule’s wrong.\(^2\)
A few years later, the controversy between Joule and Kelvin was redeemed in favor of the latter, thanks to the experiments of French military engineer Nicolas Léonard Sadi Carnot (1796–1832). The work of Carnot began in France several years before Joule and Kelvin’s time.\(^3\) At that time, the importance of steam engines was growing for industrial applications, but a theoretical perspective was lacking. Carnot was convinced that a scientific understanding of heat engines was necessary to improve their efficiency.
- Either because we don’t really have ideal gases, or because we are unable to construct mechanical devices without loss, or in general because of other experimental factors︎
- Interestingly enough, both Joule and Lord Kelvin are now recognized as key figures in the development of thermodynamics and science in general. So much so, that the energy unit and the temperature unit in the SI system are named after them.
- Carnot’s lone book, the Réflexions sur la Puissance Motrice du Feu (“Reflections on the Motive Power of Fire”) was published in France in 1824, the same year Kelvin was born and just 6 years after Joule’s birth.
- 5.1: Carnot Cycle
- The main contribution of Carnot to thermodynamics is his abstraction of the steam engine’s essential features into a more general and idealized heat engine. The definition of Carnot’s idealized cycle is as follows:
- 5.2: Energy, Heat, and Work in the Carnot Cycle
- Summarizing the results of the previous sections, the total amount of energy for a Carnot cycle is:
- 5.3: Efficiency of a Carnot Cycle
- The efficiency (ε) of a cycle is defined as the ratio between the absolute value of the work extracted from the cycle (|WTOT|) and the heat that gets into the system (|Qh|).


