Electronic Configuration of Elements
- Page ID
- 32707
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the rules for filling electrons in atomic orbitals -- Pauli exclusion principle and Hund's rule.
- Fill electrons in atomic orbitals--Aufbau process.
- Explain the arrangement of elements in terms of quantum numbers.
- Explain the systematic variation of element properties.
Mendeleev noticed the recurrence of properties of elements as the atomic weight increased, and he invented the Periodic Table of Elements, which is a useful tool for organizing and correlating chemical and physical properties of chemical elements. Today, the most popular Periodic Table form is shaped by results of quantum theory.
Quantum theory rationalized the existence of and arrangement of all elements in today's Periodic Table. It has also been applied to explain their chemical properties.
Energy Levels in Many-Electron Atoms
In order to fill the electrons in various atomic orbitals, we need to know how the energy levels vary as the nuclear charge increases. For hydrogen-like atoms, the approximate energy levels are as indicated below:
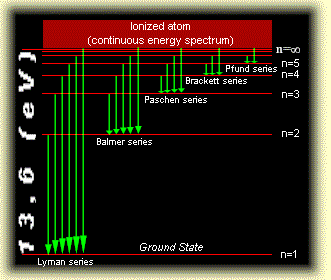
Energy levels of \(\ce{H}\)-like atoms
:::::: : ::: ::::: :::::::
4s4p4d4f - --- ----- -------
3s3p3d - --- -----
2s2p - ---
(a large gap)
1s -
|
The shielding effect and electron-electron interactions cause the energy levels of subshells such as 2s & 2p to be different from those of \(\ce{H}\)-like atoms. This is done by treating the electron shield cores as a proton but the core has an effective nuclear charge Z.
 For the \(\ce{H}\)-like atoms, energy levels for 2s, 2p stay the same, but the separation between 2s and 2p energy levels increases as the atomic number (Z) increases. Similar situations happen for 3s, 3p, and 3d energy levels. The energy diagrams of \(\ce{H}\), \(\ce{Li}\) & \(\ce{K}\) are used to illustrate this point. The color diagram is from a Hyperion website discussing quantum numbers and structure of atoms.
For the \(\ce{H}\)-like atoms, energy levels for 2s, 2p stay the same, but the separation between 2s and 2p energy levels increases as the atomic number (Z) increases. Similar situations happen for 3s, 3p, and 3d energy levels. The energy diagrams of \(\ce{H}\), \(\ce{Li}\) & \(\ce{K}\) are used to illustrate this point. The color diagram is from a Hyperion website discussing quantum numbers and structure of atoms.
| Variation of energy levels for atomic orbitals of some elements | |||||||
|---|---|---|---|---|---|---|---|
| \(\ce{H}\) _2s_ _ _2p _ 1s |
\(\ce{Li}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{Be}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{B}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{C}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{N}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{O}\) _ _ _ 2p _ 2s _ 1s |
\(\ce{F}\) _ _ _ 2p _ 2s _ 1s |
Understanding how the energy levels vary is the key to the Aufbau process, because Electrons tend to occupy the lowest energy level available. But before we talk about the Aufbau process, we need to be aware of the Pauli exclusion principle and the Hund's rule.
The Pauli Exclusion Principle
The Pauli exclusion principle suggests that only two electrons with opposite spin can occupy an atomic orbital. Stated another way, no two electrons have the same 4 quantum numbers n, l, m, s. Pauli's exclusion principle can be stated in some other ways, but the idea is that energy states have limited room to accommodate electrons. A state accepts two electrons of different spins.
In applying this rule, you should realize that an atomic orbital is an energy state.
Hund's Rule
Hund's rule suggests that electrons prefer parallel spins in separate orbitals of subshells. This rule guides us in assigning electrons to different states in each sub-shell of the atomic orbitals. In other words, electrons fill each and all orbitals in the subshell before they pair up with opposite spins.
Pauli exclusion principle and Hund's rule guide us in the Aufbau process, which is figuring out the electron configurations for all elements.
The Aufbau Procedure
The Aufbau procedure (filling order of atomic orbitals) is used to work out the electron configurations of all atoms. However, modification should be made by applying Hund's rule to be discussed in the next section.
The Aufbau procedure is based on a rough energy levels diagram of many-electron atoms as shown below:
7s |~ 5d |----- 5f |_______
Actanides
6s |_ 6p |~~~ 5d |===== 4f |=======
Lanthanides
5s |_ 5p |~~~ 4d |-----
Note the trend 5s 4d 5p
& develope a pattern
4s |_ 4p |~~~ 3d |-----
Transition elements
3s |_ 3p |---
2s |_ 2p |---
1s |-
The fine difference between energy levels cannot be adequately shown.
You've learned various techniques to work out the electronic configurations of elements. Here is yet another form of an energy level diagram:
7p _ _ _
7s _ 5f - - - - - - - 6d ~ ~ ~ ~ ~
6p _ _ _
6s _ 4f - - - - - - - 5d ~ ~ ~ ~ ~
5s _ 4d - - - - - 5p ~ ~ ~
4s _ 3d - - - - - 4p ~ ~ ~
3s _ 3p - - -
2s _ 2p - - -
1s _
In order to master the technique of the Aufbau procedure, you should apply Hund's rule and the Pauli exclusion principle to work out the electronic configuration for closed shells of inert gases. After you have it worked out, you may compare your result with the following. Do not try to remember it; it is important to know how to work it out.
Z= 2 10 18 36 54 86
1s2 2s22p6 3s23p6 4s23d104p6 5s24d105p6 6s24f145d106p6
He Ne Ar Kr Xe Rn
Block of Elements by Highest Occupied Atomic Orbitals
| Block of elements by last filled atomic orbitals |
|||
|---|---|---|---|
| 1s 2s 3s 4s 5s 6s 7s |
4f - - - - - 4f 5f - - - - - 5f |
3d - - - 3d 4d - - - 4d 5d - - - 5d 6d - - - 6d |
2p - 2p 3p - 3p 4p - 4p 5p - 5p 6p - 6p 7p - 7p |
The highest atomic orbitals occupied by electrons determine the properties of the elements. According to this scheme, the periodic table can be divided into s, p, d, and f blocks as seen in the table on the right.
This table shows the filling order of atomic orbitals as
1s
2s 2p
3s 3p
4s 3d 4p
5s 4d 5p
6s 4f 5d 6p
7s 5f 6d 7p
The s- and p-blocks of elements are called main group elements. The d-block elements are called transition elements The f-block elements are called the inner transition elements.
In an ordinary periodic table, the s, p, and d block elements are in the main body of the Periodic Table, whereas the f block elements are placed below the main body. If we placed them on the same period where they belong, the Periodic Table would be too long for the screen to accommodate. Thus, we keep the Periodic Table in the usual (long) form.
Special Electronic Configurations
When two electrons occupy the same orbital, they not only have different spins (Pauli exclusion principle), the pairing raises the energy slightly. On the other hand, a half filled subshell and a full filled subshell lower the energy, gaining some stability. Bearing this in mind, you will be able to understand why we have the following special electronic configurations.
| \(\ce{Cr\: [Ar]}4s^1 3d^5\) | <=All s and d subshells are half full |
| \(\ce{Cu\: [Ar]}4s^1 3d^{10}\) | <=Prefers a filled d subshell, leaving s with 1 |
| \(\ce{Nb\: [Kr]}5s^1 4d^4\) | <=5s and 4d energy levels are close |
| \(\ce{Mo\: [Kr]}5s^1 4d^5\) | Similar to \(\ce{Cr}\) above |
| \(\ce{Tc\: [Kr]}5s^2 4d^5\) | (Not special, but think of Hund's rule) |
| \(\ce{Ru\: [Kr]}5s^1 4d^7\) | <= Only 1 5s electron |
| \(\ce{Rh\: [Kr]}5s^1 4d^8\) | <= In both |
| \(\ce{Pd\: [Kr]}5s^0 4d^{10}\) | <= Note filled 4d and empty 5s |
| \(\ce{Ag\: [Kr]}5s^1 4d^{10}\) | <= Partially filled 5s, but filled d |
For CHEM120 students, you are not required to remember the special ones, but you should take notice of the electronic configurations of \(\ce{Cr}\) and \(\ce{Cu}\) to realize the gaining of stability due to half and full filled subshells.
Confidence Building Questions
- For the \(\ce{H}\)-like atom, which subshell has the highest energy level?
4f, 3d, 2p, 1sHint: 4f
Skill:
Describe the energy levels of hydrogen atoms. - For an element with atomic number 20, which is the last or highest occupied subshell of atomic orbitals?
1s 2s 2p 3s 3p 3d 4s 5sDiscussion:
The element is Calcium, \(\ce{Ca}\). - How many electrons are required to fill all the following sub shells?
1s 2s 2p 3s 3p 4s 3d 4pDiscussion:
The element is Krypton, \(\ce{Kr}\). - Which of the following two electronic configurations is more stable?
a \(\ce{[Ar]}4s^1 3d^5\)
b \(\ce{[Ar]}4s^2 3d^4\)Discussion:
Check the electronic configuration for \(\ce{Cu}\) and \(\ce{Cr}\). - Which of the following two electronic configurations is more stable?
a \(\ce{[Ar]}4s^2 3d^9\)
b \(\ce{[Ar]}4s^1 3d^{10}\)Discussion:
This is the electronic configuration for copper. - Choose the electronic configuration for palladium, \(\ce{Pd}\) (Z = 46).
a \(\ce{[Kr]}5s^1 4d^7\)
b \(\ce{[Kr]}5s^1 4d^8\)
c \(\ce{[Kr]}5s^0 4d^{10}\)
d \(\ce{[Kr]}5s^1 4d^{10}\)Discussion:
Excellent. This is a special case. One can argue that the filled 4d subshell has lower energy than 5s2 4d8.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)


