Fine Structure
- Page ID
- 96023
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)From classical electrodynamics, a rotating electrically charged body creates a magnetic dipole with magnetic poles of equal magnitude but opposite polarity. This analogy holds as an electron indeed behaves like a tiny bar magnet. In the classical model, the electron (Bohr atom model) is moving along a closed path. A revolving electron is equivalent to a current loop (Figure \(\PageIndex{1a}\)).
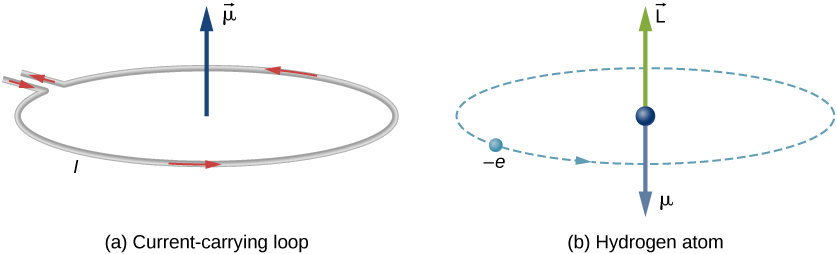
The current arising from the orbiting of the electron around the nucleus is
\[I = \dfrac{e}{T}\]
where \(T\) is the orbital period or revolution. We can define the velocity then as
\[v=\dfrac{2πr}{T} \]
where \(r\) is the radius of orbit. The "orbital" magnetic (dipole) moment is then
\[\vec{\mu}_L = \dfrac{\vec{\mu}e}{T} (π r^2) \label{eq1}\]
and the angular momentum associated with that orbital motion is
\[ \begin{align} L &= m_e v_r \\[4pt] &= \dfrac{m_e 2\pi r^2}{T} \label{eq2} \end{align}\]
and \(m_e\) is the electron rest mass.
Next we combine Equation \ref{eq1} and \ref{eq2} to get how the orbital angular momentum induced a magnetic moment (in vector form)
\[ \color{red} \vec{\mu}_L = - \dfrac{e}{2m_e} \vec{L} \label{classical}\]
For a more detailed derivation of Equation \ref{classical}, look here. We can glean two important feature from Equation \ref{classical}:
- If the electron has zero orbital angular momentum, it will have a zero-amplitude orbital magnetic moment.
- The orientation of the orbital angular moment is parallel to the angular momentum.
The Quantum Orbital Magnetic Dipole Moment
Equation \ref{classical} is applicable to classical systems and since \(\vec{L}\) can have any values (amplitude and orientation), so can the magnetic moment. That is a different story in the quantum world. where the orbital angular momentum is quantized and hence so is \(\mu_L\). The amplitude of is \(\vec{L}\) represented by the \(\hat{L}^2\) operator and the projection of \(\vec{L}\) on the z-axis is represented by \(\hat{L}_z\) operator.
\[\hat{L^2} | \psi \rangle = \ell(\ell+1) \hbar^2 | \psi \rangle\]
and
\[\hat{L}_z | \psi \rangle = m_l \hbar | \psi \rangle\]
So we obtain the eigenvalues \(µ_{L_z}\) of z-component of the magnetic moment
\[µ_{L_z} = −\dfrac{ e}{2m_e} \hbar m_l \label{eq5}\]
It is usual to express the magnetic moment (Equation \ref{eq5}) in terms the Bohr magneton \(\mu_B\)
\[µ_{L_z} = −µ_B \,m_l\]
where \(µ_B\) is the Bohr Magneton:
\[µ_B = \dfrac{e\hbar}{2m_e}\]
The Bohr magneton is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by either its orbital angular momentum and (and spin as discussed below) and is numerically
\[\begin{align} µ_B &= 9.273 \times 10^{-24}\, J/K \\[4pt] &= 5.656 \times 10^{-5}\, eV/T \\[4pt] &= 1.4 \times 10^{10}\, Hz/T \,(T \,= \,Tesla) \end{align}\]
Coupling Orbital Magnetic Dipole Moment to an External Magnetic Field
When a magnetic field is applied to the electron, then the energies of the eigenstates will depends on the magnitude \(B\) of the applied magnetic field \(\vec{B}\) via
\[ \begin{align} E_B &= −µ_{L_z} \, B \\[4pt] &= µ_B\, m_l\, B \end{align}\]
and the total energy of that state is
\[\begin{align} E &= E_0 + E_B \\[4pt] &= E_0 + µ_B\, m_l\, B \end{align}\]
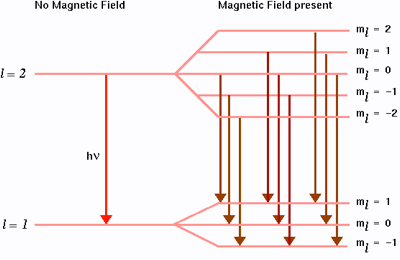
Hence, the energy levels having a angular momentum with quantum number \(\ell\) are split into \(2\ell + 1\) new levels (Figure \(\PageIndex{2}\)):
This effect is the "ordinary" or "normal" Zeeman Effect.
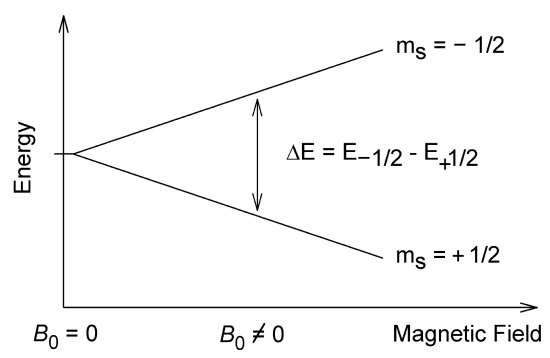
Coupling Spin Magnetic Dipole Moment to an External Magnetic Field
The hydrogen ground state (\(m_l = 0\)) is uninfluenced in Figure \(\PageIndex{1}\). However, we know that a H atom is paramagnetic; this is because of electron spin. Now we consider the spin in classical mechanics as rotating around the axis electron. We also find here
\[µ_{S_z} = −g_S\, µ_B \, m_s \]
\[E_B = g_S\, µ_B\, m_s\, B\]
The so-called gyromagnetic factor \(g_S\) is obtained from the relativistic Dirac equation and experiments quantify it at
\[g_S = 2.00231930438(6)\]
The value
\[g_S\, µ_B ≈ 28\, GHz/T\]
shows us which energy state is higher according to electron spin interaction with magnetic field. Since now it's possible to produce magnetic fields with the strength of a few Teslas, we expect to detect transitions in GHz region (microwaves) when applying such fields. That's why Electron-Spin-Resonance (ESR) Spectroscopy is involved with microwaves. When applying NMR method we have a deal with MHz region. We will discuss it in more detail later.


http://www.pci.tu-bs.de/aggericke/Le...SR_NMR/NMR.htm