Hydrophobic Interactions
- Page ID
- 1506
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydrophobic interactions describe the relations between water and hydrophobes (low water-soluble molecules). Hydrophobes are nonpolar molecules and usually have a long chain of carbons that do not interact with water molecules. The mixing of fat and water is a good example of this particular interaction. The common misconception is that water and fat doesn’t mix because the Van der Waals forces that are acting upon both water and fat molecules are too weak. However, this is not the case. The behavior of a fat droplet in water has more to do with the enthalpy and entropy of the reaction than its intermolecular forces.
Causes of Hydrophobic Interactions
American chemist Walter Kauzmann discovered that nonpolar substances like fat molecules tend to clump up together rather than distributing itself in a water medium, because this allow the fat molecules to have minimal contact with water.
The image above indicates that when the hydrophobes come together, they will have less contact with water. They interact with a total of 16 water molecules before they come together and only 10 atoms after they interact.
Thermodynamics of Hydrophobic Interactions
When a hydrophobe is dropped in an aqueous medium, hydrogen bonds between water molecules will be broken to make room for the hydrophobe; however, water molecules do not react with hydrophobe. This is considered an endothermic reaction, because when bonds are broken heat is put into the system. Water molecules that are distorted by the presence of the hydrophobe will make new hydrogen bonds and form an ice-like cage structure called a clathrate cage around the hydrophobe. This orientation makes the system (hydrophobe) more structured with an decrease of the total entropy of the system; therefore \( \Delta S < 0\).
The change in enthalpy (\( \Delta H \)) of the system can be negative, zero, or positive because the new hydrogen bonds can partially, completely, or over compensate for the hydrogen bonds broken by the entrance of the hydrophobe. The change in enthalpy, however, is insignificant in determining the spontaneity of the reaction (mixing of hydrophobic molecules and water) because the change in entropy (\( \Delta S \)) is large.
According to the Gibbs Energy formula
\[ \Delta G = \Delta H - T\Delta S \label{eq1}\]
with a small unknown value of \(\Delta H\) and a large negative value of \(\Delta{S} \), the value of \(\Delta G\) will turn out to be positive. A positive \(\Delta G\) indicates that the mixing of the hydrophobe and water molecules is not spontaneous.
Formation of Hydrophobic Interactions
The mixing hydrophobes and water molecules is not spontaneous; however, hydrophobic interactions between hydrophobes are spontaneous. When hydropobes come together and interact with each other, enthalpy increases ( \( \Delta H \) is positive) because some of hydrogen bonds that form the clathrate cage will be broken. Tearing down a portion of the clathrate cage will cause the entropy to increase ( \( \Delta S \) is positive), since forming it decreases the entropy.
According to the Equation \(\ref{eq1}\)
- \( \Delta{H} \) = small positive value
- \( \Delta{S} \) = large positive value
Result: \( \Delta{G} \) is negative and hence hydrophobic interactions are spontaneous.
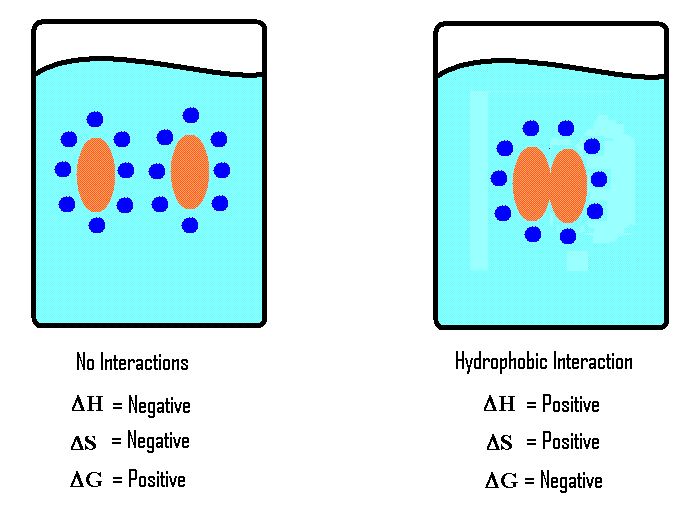
Strength of Hydrophobic Interactions
Hydrophobic interactions are relatively stronger than other weak intermolecular forces (i.e., Van der Waals interactions or Hydrogen bonds). The strength of Hydrophobic Interactions depend on several factors including (in order of strength of influence):
- Temperature: As temperature increases, the strength of hydrophobic interactions increases also. However, at an extreme temperature, hydrophobic interactions will denature.
- Number of carbons on the hydrophobes: Molecules with the greatest number of carbons will have the strongest hydrophobic interactions.
- The shape of the hydrophobes: Aliphatic organic molecules have stronger interactions than aromatic compounds. Branches on a carbon chain will reduce the hydrophobic effect of that molecule and linear carbon chain can produce the largest hydrophobic interaction. This is so because carbon branches produce steric hindrance, so it is harder for two hydrophobes to have very close interactions with each other to minimize their contact to water.
Biological Importance of Hydrophobic Interactions
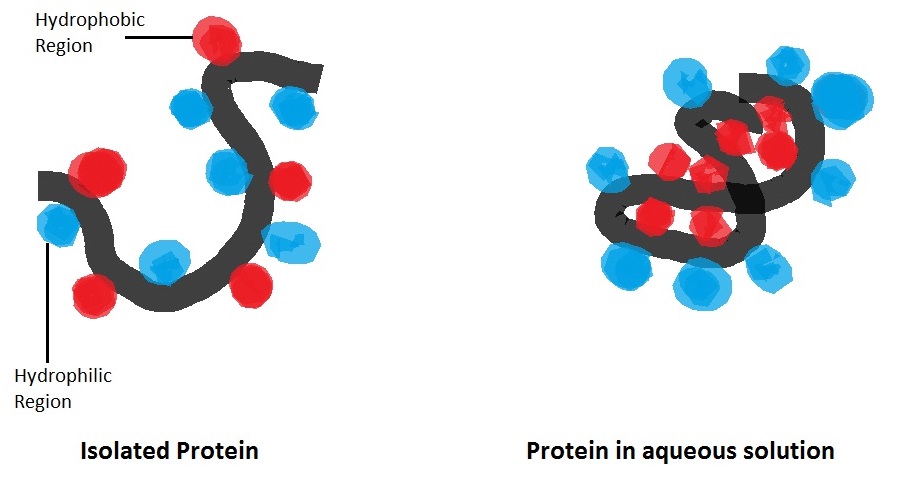
Hydrophobic Interactions are important for the folding of proteins. This is important in keeping a protein stable and biologically active, because it allow to the protein to decrease in surface are and reduce the undesirable interactions with water. Besides from proteins, there are many other biological substances that rely on hydrophobic interactions for its survival and functions, like the phospholipid bilayer membranes in every cell of your body!
References
- Atkins, Peter and Julio de Paula. Physical Chemistry for the Life Sciences. Oxford, UK: Oxford University Press. 2006. 95.
- Chang, Raymond. Physical Chemistry for the Biosciences. Sausalito, CA: Edwards Brothers, Inc. 2005. 508-510.
- Garrett, Reginald H. and Charles M. Grisham. Biochemistry. Belmont, CA: Thomas Brooks/Cole. 2005. 15.