12.5: Molecules can be Represented by Reducible Representations
- Page ID
- 13488
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In addition to operators, we can define properties of molecules using a matrix representation. Before making the matrix, we need to carefully choose a basis set that defines the information we want to extract For example, let's say we want to know the symmetry of the valence \(s\) orbitals in ammonia, \(\sf NH_3\), which is in the \(C_{3v}\) point group. We will select a basis \(\begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix}\) that consists of the valence s orbitals on the nitrogen and the three hydrogen atoms. We need to consider what happens to this basis when it is acted on by each of the symmetry operations in the \(C_{3v}\) point group, and determine the matrices that would be required to produce the same effect. The basis set and the symmetry operations in the \(C_{3v}\) point group are summarized in the figure below.
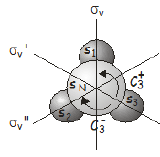
The effects of the symmetry operations on our chosen basis are as follows:
\[\begin{array}{ll} E & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \\ C_3^+ & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_2, s_3, s_1 \end{pmatrix} \\ C_3^- & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_3, s_1, s_2 \end{pmatrix} \\ \sigma_v & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_1, s_3, s_2 \end{pmatrix} \\ \sigma_v' & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_2, s_1, s_3 \end{pmatrix} \\ \sigma_v'' & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \rightarrow \begin{pmatrix} s_N, s_3, s_2, s_1 \end{pmatrix} \end{array} \label{10.1} \]
By inspection, the matrices that carry out the same transformations are:
\[\begin{array}{ll} \Gamma(E) & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix}\begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 1 & 0 & 0 \\ 0 & 0 & 1 & 0 \\ 0 & 0 & 0 & 1 \end{pmatrix} = \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \\ \Gamma(C_3^+) & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 0 & 0 & 1 \\ 0 & 1 & 0 & 0 \\ 0 & 0 & 1 & 0 \end{pmatrix} = \begin{pmatrix} s_N, s_2, s_3, s_1 \end{pmatrix} \\ \Gamma(C_3^-) & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 0 & 1 & 0 \\ 0 & 0 & 0 & 1 \\ 0 & 1 & 0 & 0 \end{pmatrix} = \begin{pmatrix} s_N, s_3, s_1, s_2 \end{pmatrix} \\ \Gamma(\sigma_v) & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 1 & 0 & 0 \\ 0 & 0 & 0 & 1 \\ 0 & 0 & 1 & 0 \end{pmatrix} = \begin{pmatrix} s_N, s_1, s_3, s_2 \end{pmatrix} \\ \Gamma(\sigma_v') & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 0 & 1 & 0 \\ 0 & 1 & 0 & 0 \\ 0 & 0 & 0 & 1 \end{pmatrix} = \begin{pmatrix} s_N, s_2, s_1, s_3 \end{pmatrix} \\ \Gamma(\sigma_v'') & \begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix} \begin{pmatrix} 1 & 0 & 0 & 0 \\ 0 & 0 & 0 & 1 \\ 0 & 0 & 1 & 0 \\ 0 & 1 & 0 & 0 \end{pmatrix} = \begin{pmatrix} s_N, s_3, s_2, s_1 \end{pmatrix} \end{array} \label{10.2} \]
These six matrices therefore form a reducible representation for the \(C_{3v}\) point group in the \(\begin{pmatrix} s_N, s_1, s_2, s_3 \end{pmatrix}\) basis as we will see in the next chapter that these matrices reduce down to the irreducible representations found in the character tables. These reducible representations multiply together according to the group multiplication table and satisfy all the requirements for a mathematical group.
We choose different basis sets to extract different properties of molecules. For example, we could include representations of the valence \(p\) orbitals in N in our basis set to obtain the structure and symmetry of the molecular orbitals for ammonia. To understand understand the molecular motions of ammonia (translates, rotates, and vibrates), we could place a \(x\), \(y\), and \(z\) unit vectors on each atom to represent the their motion, and then construct our matrices.

