Oxidation of Organic Molecules by KMnO4
- Page ID
- 15387
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Potassium permanganate, KMnO4, is a powerful oxidizing agent, and has many uses in organic chemistry.
Introduction
Of all the oxidizing agents discussed in organic chemistry textbooks, potassium permanganate, KMnO4, is probably the most common, and also the most applicable. As will be shown below, KMnO4 can be utilized to oxidize a wide range of organic molecules. The products that are obtained can vary depending on the conditions, but because KMnO4 is such a strong oxidizing agent, the final products are often carboxylic acids.
The half-reaction and oxidation potential
Mn(VII) is reduced under acidic conditions to Mn(IV) or Mn(II) according to the half-reactions shown below, with the indicated cell potentials1
\[MnO_4^- + 4H^+ + 3e^- \rightarrow MnO_2 + 2H_2O\;\;\;\;E^o = 1.68\,V\]
\[MnO_4^- + 8H^+ + 5e^- \rightarrow Mn^{2+} + 4H_2O\;\;\;\;E^o = 1.5\, V\]
\[MnO_4^- + 2H_2O + 3e^- \rightarrow Mn^{2+} + 4OH^-\;\;\;\;E^o = 0.6\, V\]
General Reactivity with Organic Molecules
KMnO4 is able to oxidize carbon atoms if they contain sufficiently weak bonds, including
- Carbon atoms with \(\pi\) bonds, as in alkenes and alkynes
- Carbon atoms with weak C-H bonds, such as
- C-H bonds in the alpha-positions of substituted aromatic rings
- C-H bonds in carbon atoms containing C-O bonds, including alcohols and aldehydes
- Carbons with exceptionally weak C-C bonds such as
- C-C bonds in a glycol
- C-C bonds next to an aromatic ring AND an oxygen
KMnO4 also oxidizes phenol to para-benzoquinone.
Examples of carbons that are not oxidized
- Aliphatic carbons (except those alpha to an aromatic ring, as above)
- Aromatic carbons (except phenol, as above)
- Carbons without a C-H bond, except as in (3) above
Exhaustive oxidation of organic molecules by KMnO4 will proceed until the formation of carboxylic acids. Therefore, alcohols will be oxidized to carbonyls (aldehydes and ketones), and aldehydes (and some ketones, as in (3) above) will be oxidized to carboxylic acids.
Reactions with Specific Functional Groups
Using the principles above, we expect KMno4 to react with alkenes, alkynes, alcohols, aldehydes and aromatic side chains. Examples are provided below. It is easiest to start at the top.
Aldehydes
Aldehydes RCHO are readily oxidized to carboxylic acids.
Unless great efforts are taken to maintain a neutral pH, KMnO4 oxidations tend to occur under basic conditions. In fact, the most effective conditions for aldehyde oxidation by KMnO4 involves t-butanol as solvent with a NaH2PO4 buffer.2 The reactions above are deliberately not balanced equations. Balancing the reactions would involve using the methods learned in general chemistry, requiring half reactions for all processes.
Alcohols
Primary alcohols such as octan-1-ol can be oxidized efficiently by KMnO4, in the presence of basic copper salts.3 However, the product is predominantly octanoic acid, with only a small amount of aldehyde, resulting from overoxidation.
Although overoxidation is less of a problem with secondary alcohols, KMnO4 is still not considered generally well-suited for conversions of alcohols to aldehydes or ketones.
Alkenes4
Under mild conditions, potassium permanganate can effect conversion of alkenes to glycols. It is, however, capable of further oxidizing the glycol with cleavage of the carbon-carbon bond, so careful control of the reaction conditions is necessary. A cyclic manganese diester is an intermediate in these oxidations, which results in glycols formed by syn addition.
With addition of heat and/or more concentrated KMnO4, the glycol can be further oxidized, cleaving the C-C bond.
More substituted olefins will terminate at the ketone
Oxidative cleavage of the diol can be carried out more mildly by using IO4 as the oxidant.
The cleavage of alkenes to ketones/carboxylic acids can be used to determine the position of double bonds in organic molecules.5
Alkynes4
Instead of bis-hydroxylation that occurs with alkenes, permanganate oxidation of alkynes initially leads to the formation of diones.
Under harsher conditions, the dione is cleaved to form two carboxylic acids.
Aromatic side-chains6
Treatment of an alkylbenzene with potassium permanganate results in oxidation to give the benzoic acid.
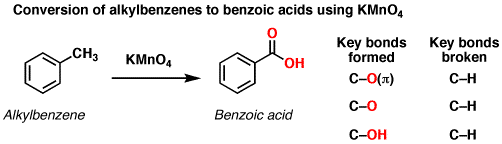
The position directly adjacent to an aromatic group is called the “benzylic” position.
The reaction only works if there is at least one hydrogen attached to the carbon. However, if there is at least one hydrogen, the oxidation proceeds all the way to the carboxylic acid.
Examples:

Notes: Note that in example 2 the extra carbons are cleaved to give the same product as in example 1. And in example 3, two benzoic acids are formed. Finally, when no hydrogens are present on the benzylic carbon, no reaction occurs (example 4).
The oxidation of alkyl side-chains to form benzoic acids was historically used in qualitative analysis to determine the positions of alkyl groups in substituted aromatic systems. Alkyl-substituted rings can be coverted to poly-acids, which can be distinguished on the basis of their pKas
Additional Reading
- Oxidation by Chromic Acid (H2CrO4)
- Ozonolysis
- Oxidative cleavage of double bonds
- Oxidation of alkenes
Sources
- http://www.epa.gov/ogwdw/mdbp/pdf/alter/chapt_5.pdf
- Abiko, Atsushi; Roberts, John C.; Takemasa, Toshiro; Masamune, Satoru, Tetrahedron Letters (1986), 27(38), 4537-40
- Jefford, Charles W.; Wang, Ying, Journal of the Chemical Society, Chemical Communications (1988), (10), 634-5.
- Carey, F.A.; Sundberg, R. J. Advanced Organic Chemistry
- Downing, Donald T.; Greene, Richard S., Journal of Investigative Dermatology (1968), 50(5), 380-6.
- http://www.masterorganicchemistry.co...boxylic-acids/

